Abstract
Missense mutations in the α-synuclein gene cause familial Parkinson’s disease (PD), and α-synuclein is a major component of Lewy bodies (LBs) in sporadic PD, dementia with LBs (DLB), and the LB variant of Alzheimer’s disease (AD). To determine whether α-synuclein is a component of LBs in familial AD (FAD) patients with known mutations in presenilin (n = 65) or amyloid precursor protein (n = 9) genes, studies were conducted with antibodies to α-, β-, and γ-synuclein. LBs were detected with α- but not β- or γ-synuclein antibodies in 22% of FAD brains, and α-synuclein-positive LBs were most numerous in amygdala where some LBs co-localized with tau-positive neurofibrillary tangles. As 12 (63%) of 19 FAD amygdala samples contained α-synuclein-positive LBs, these inclusions may be more common in FAD brains than previously reported. Furthermore, α-synuclein antibodies decorated LB filaments by immunoelectron microscopy, and Western blots revealed that the solubility of α-synuclein was reduced compared with control brains. The presence of α-synuclein-positive LBs was not associated with any specific FAD mutation. These studies suggest that insoluble α-synuclein aggregates into filaments that form LBs in many FAD patients, and we speculate that these inclusions may compromise the function and/or viability of affected neurons in the FAD brain.
Although Lewy bodies (LBs) are the hallmark lesions of Parkinson’s disease (PD), they also occur frequently in the brains of many patients with autopsy-confirmed Alzheimer’s disease (AD) for reasons that are poorly understood. Indeed, immunohistochemical studies have demonstrated the presence of widespread cortical LBs in a subtype of AD known as the LB variant of AD (LBVAD). 1-4 Although it is possible that LBs are a nonspecific outcome of end-stage AD, LBs may reflect the co-occurrence of PD in a subset of AD patients. Alternatively, the accumulation of LBs in the AD brain may result from the pathogenic mechanisms that underlie AD. Thus, although tau-rich neurofibrillary tangles (NFTs) and senile plaques (SPs) formed from Aβ-rich amyloid fibrils are the signature lesions of AD, unknown genetic or epigenetic factors may predispose neurons to accumulate LBs during the progression of AD in a subset of affected patients.
Interest in elucidating the mechanisms that lead to LB formation has intensified after the discovery that missense mutations in the α-synuclein gene on chromosome 4q21-23 cause familial PD. 5, 6 These data strongly implicate α-synuclein in the sequence of abnormal events that result in the formation of LBs, and this notion is supported by other studies demonstrating that α-synuclein is a major building block of LBs in sporadic PD, dementia with LBs (DLB), and LBVAD. 7-10 As LBs purified from DLB brains contain full-length, partially truncated and insoluble high molecular weight aggregates of α-synuclein, 9 a reduction in the solubility of α-synuclein may play a role in this process. 9 Thus, neurodegenerative diseases characterized by the accumulation of LBs may result from alterations in wild-type α-synuclein as well as from mutant forms of this protein. Although two other proteins (ie, β- and γ-synuclein) are partially homologous with α-synuclein, they have not been detected in LBs, 9 and it is uncertain whether they play a role in other neurodegenerative disorders.
Evidence that LBs may result from pathogenic mutations other than those affecting the α-synuclein gene has emerged from studies showing that LBs are present in the brains of some patients with familial AD (FAD) and some Down’s syndrome patients. 11-14 Studies of the prevalence and composition of LBs in FAD are important for elucidating how mutations in the genes that cause hereditary forms of AD lead to the accumulation of α-synuclein into filamentous inclusions that may compromise the function and viability of affected neurons in FAD and other neurodegenerative disorders. However, cortical LBs are difficult to detect even when anti-ubiquitin antibodies are used to label these inclusions by immunohistochemistry. As ubiquitin is present in a variety of brain lesions, it is not a specific marker of LBs. For example, it is not always possible to distinguish ubiquitin-positive LBs and NFTs from one another. Thus, the demonstration that antibodies to α-synuclein distinguish LBs from NFTs and a number of other morphologically similar lesions by immunohistochemistry 7-10 prompted us to investigate the prevalence and composition of LBs in the brains of patients with FAD.
Materials and Methods
Tissue Samples
Tissue Samples from FAD Cases
Previously characterized tissue samples were available for the studies described here, and they came from 58 FAD patients with mutations in the presenilin (PS)-1 gene, 9 FAD patients with mutations in the amyloid precursor protein (APP) gene, and 7 FAD patients with a mutation in the PS-2 gene. 15-21 The pathological diagnosis of definite AD was confirmed in the postmortem brains of these FAD cases using recently revised neuropathological criteria. 22 Five PS-1 cases were biopsy specimens from patients with early symptoms. Although nearly all of the FAD patients had advanced dementia at death, one patient with an A246E PS-1 mutation had preclinical AD, and when this patient died of a myocardial infarction at age 51, his brain showed numerous Aβ plaques, with little neurofibrillary degeneration (Braak stage I). 23 The brain samples that were available for study here (see Table 1 ▶ ) came from the following regions: substantia nigra (n = 15), locus ceruleus (n = 12), amygdala (n = 19), periamygdaloid entorhinal cortex (n = 18), parahippocampal gyrus (at the level of the lateral geniculate nucleus; n = 18), cingulate gyrus (n = 25), middle frontal cortex (n = 66), and cerebellum (n = 11). We also examined tissue blocks from organs other than the brain (ie, lung, heart, thyroid, liver, pancreas, kidney, and adrenal gland) from one FAD case with an M146L PS-1 mutation. The apolipoprotein E (ApoE) genotype of 62 of these FAD cases was determined using methods described in previous studies. 15-21 All except 16 cases had the ApoE-ɛ3/3 genotype (four were ApoE-ɛ2/3, 11 were ApoE-ɛ3/4, and one had the ApoE-ɛ4/4 genotype).
Table 1.
Summary of Data
| Gene | Mutation | Number of cases | Age/ Gender | Average duration | AMYG | CG | MFG | SN | PAC | PHG LGN | LC | CBM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS-1 | C410Y | 8 | 58 /4 M | 14 | 3 /4 | 1 /4 | 2 /7 | 0 /4 | 3 /4 | 2 /6 | 0 /3 | 0 /3 |
| PS-1 | A246E | 13 | 60 /9 M | 11 | 1 /6 | 0 /7 | 2 /10 | 0 /8 | 1 /6 | 1 /8 | 0 /6 | 0 /6 |
| PS-1 | E280A | 8 | 56 /3 M | 8 | 1 /8 | 1 /8 | ||||||
| PS-1 | M146L | 6 | 51 /2 M | 10 | 1 /1 | 1 /2 | 1 /4 | 1 /2 | 1 /1 | 1 /2 | 0 /2 | 0 /1 |
| PS-1 | M146I | 4 | NA | NA | 0 /4 | |||||||
| PS-1 | H163R | 4 | 55 /2 M | 12 | 0 /4 | |||||||
| PS-1 | E120D | 2 | 57 /2 M | 13 | 2 /2 | 0 /2 | 2 /2 | |||||
| PS-1 | E120K | 2 | 49 /2 M | 10 | 0 /2 | |||||||
| PS-1 | M139I | 2 | 42 /2 M | 4 | 0 /2 | |||||||
| PS-1 | I143F | 2 | 61 /0 M | 6 | 0 /2 | |||||||
| PS-1 | G209V | 2 | 48 /1 M | 7 | 2 /2 | 0 /2 | 0 /2 | |||||
| PS-1 | A260V | 2 | 58 /0 M | 1 /1 | 1 /1 | |||||||
| PS-1 | L250S | 2 | 53 /0 M | 7 | 1 /2 | |||||||
| PS-1 | L286V | 1 | 61 /1 M | 8 | 1 /1 | 1 /1 | 1 /1 | 1 /1 | 1 /1 | 0 /1 | 1 /1 | 0 /1 |
| PS-2 | N141I | 7 | 60 /4 M | 14 | 0 /1 | 1 /1 | 1 /7 | 0 /1 | ||||
| APP | V7171 | 6 | 61 /1 M | 8 | 0 /6 | |||||||
| APP | V717G | 1 | 61 /1 M | 21 | 0 /1 | |||||||
| APP | V717F | 2 | 51 /0 M | 7 | 1 /1 | 1 /2 | 0 /2 | 0 /1 | 0 /1 | |||
| TOTAL | FAD | 74 | 56 /34 M | 10.3 | 12 /19 | 6 /25 | 9 /66 | 2 /15 | 8 /18 | 4 /18 | 1 /12 | 0 /11 |
| Sporadic AD | NA | 12 | 74 /6 M | 9 | 2 /12 | 1 /10 | 0 /10 | 0 /10 | 1 /12 | 0 /10 | 0 /5 | 0 /8 |
| DLB | NA | 8 | 72 /5 M | 8 | 6 /6 | 6 /6 | 6 /8 | 8 /8 | 6 /6 | 7 /8 | 1 /3 | 0 /6 |
| CTRL | NA | 25 | 71 /15 M | 0 | 0 /25 | 0 /10 | 0 /25 |
Age = Age at death; Duration = Disease duration in years
Amyg, amygdala; CG, cingulate gyrus; MFG, middle frontal gyrus; SN, substantia nigra; PAC, periamygdaloid cortex; PHG LGN, parahippocampal gyrus at the level of the lateral geniculate nucleus; LC, locus ceruleus; CBM, cerebellar cortex; M, male; F, female. In each brain region the numerator indicates the number of cases with Lewy bodies. The denominator indicates the number of cases where tissue was available for examination.
Tissue Samples from Patients with Other Dementias
For comparison with the data from the FAD cases, we examined brain tissue from 8 patients who met criteria for DLB 24 and 12 patients who met criteria for definite AD. 22, 23, 25 These AD patients were considered to have sporadic disease as none had a first-degree relative with dementia.
Tissue Samples from Normal Control Cases
We examined brain tissue from 25 individuals who were documented to be cognitively normal before death by review of their medical records. None of these individuals met criteria for probable or definite AD after postmortem examination of their brains, and none of these brains had significant neuropathological abnormalities. Two control cases were unaffected members of the family with the PS-2 mutation, and one was an unaffected member of the PS-1 A246E family.
Immunohistochemistry and Anti-Synuclein Antibodies
Formalin fixed, paraffin-embedded sections cut at 6 μm were stained with a panel of previously described monoclonal antibodies (MAbs) and polyclonal antibodies 9 raised to purified LBs and HPLC-purified full-length recombinant α-, β-, and γ-synucleins. 26, 27 Immunohistochemistry was performed using the avidin-biotin complex technique with 3,3′-diaminobenzidine (DAB) as the chromogen and a modified antigen retrieval protocol followed by counterstaining with hematoxylin. 9, 28, 29 The anti-synuclein antibodies used here included MAb LB509, which is specific for α-synuclein, MAb Syn207, which recognizes only β-synuclein, and mouse polyclonal antiserum 20, which detects only γ-synuclein. Sections of cingulate gyrus and substantia nigra from a patient with DLB were used as positive control tissue. Adjacent sections stained with nonimmune serum in place of the primary antibody served as negative controls. To determine whether LBs co-localized with NFTs in the same neurons, we also performed double-label immunohistochemistry using antibodies to tau and α-synuclein using methods similar to those reported elsewhere. 9
Immunoelectron Microscopy
Immunoelectron microscopy (immuno-EM) was performed using sections of amygdala from PS-1 cases known to have numerous LBs. Tissue samples were fixed for 2 weeks in 10% formalin, embedded in paraffin, and sectioned at 6 μm, and deparaffinized sections were probed with α-synuclein antibodies to identify LBs using a two-step immunoperoxidase method with the chromogen DAB as described. 9 Small regions with immunostained LBs were excised and processed for electron microscopic examination as reported earlier. 9
Western Blot Analysis
To examine the biochemical properties of α-synuclein in LB-rich regions of the FAD brain, we performed Western blot analysis on extracts of the amygdala from three PS-1 cases (two with LBs in the amygdala) and three age-matched normal controls. The methods for extracting high-salt-soluble and Triton X-100-soluble α-synuclein from postmortem human brain tissue and for Western blot analysis were identical to those reported recently by Baba et al. 9 For formic acid extracts, pellets were resuspended in 100 μl of 70% formic acid and disrupted with three 1-second sonication bursts. Formic acid was evaporated in an Automatic Environmental SpeedVac System (Savant Instruments, Holbrook, NY). The dried pellets were resuspended in 100 μl of SDS sample buffer and heated to 100°C for 10 minutes. These extracts were centrifuged at 40,000 rpm for 30 minutes in a TL-100 rotor before being used for Western blot analysis.
Results
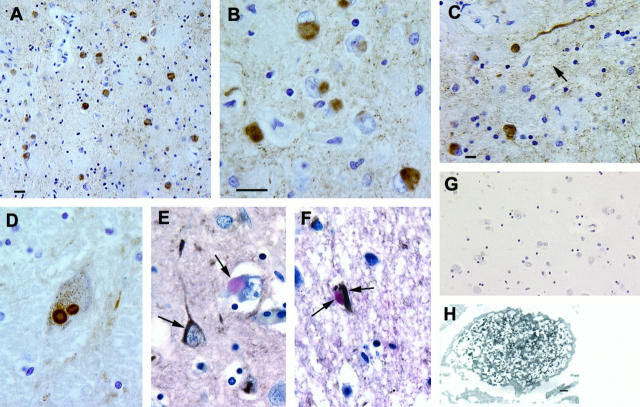
The most striking immunohistochemical observation in the FAD cases was abundance of α-synuclein-positive LBs in the amygdala of 12 of the 19 available samples (Figure 1, A and B) ▶ . Some of these LBs appeared to co-localize with NFTs in the same neurons, and this was confirmed by double-label immunohistochemistry using antibodies to tau and α-synuclein (Figure 1, E and F) ▶ . Although these α-synuclein-positive LBs most commonly appeared to be solid inclusions, occasional LBs demonstrated an immunoreactive halo with a clear core. Consistent with previous studies, 7-10 α-synuclein-immunoreactive LBs also were seen in cases of sporadic AD with LBs (ie, LBVAD cases) and DLB, but not in normal control cases (data not shown). Light cytoplasmic α-synuclein immunoreactivity was present in neurons of the amygdala in only 2 of 12 cases with sporadic AD.
Figure 1.
Low-magnification (A) and high-magnification (B) photomicrographs of numerous α-synuclein-positive LBs and occasional (arrow) Lewy neurites (C). A nigral LB stained with the α-synuclein antibody is demonstrated in D. E and F show LBs examined by double-label immunohistochemistry to demonstrate α-synuclein-positive (pink) LBs and tau-positive (brown; see arrows) NFTs in different (E) or the same (F) neurons. A section of the amygdala from a normal control contains no α-synuclein-positive LBs or other lesions (G). Immuno-EM demonstrates an α-synuclein-positive LB in an amygdala neuron of a PS-1 patient (H). LB filaments are decorated by the antibody to α-synuclein. Scale bars, 30 μm (A and G), 10 μm (C), and 20 μm (B and D–F), and 200 μm (H).
Similar appearing α-synuclein-positive LBs also were identified in the adjacent periamygdaloid cortex (in 8 of 18 FAD samples examined), the parahippocampal gyrus (in 4 of 18 FAD samples examined), the cingulate gyrus (in 6 of 25 FAD samples examined), and areas 8 and 9 of the frontal lobe (in 9 of 66 FAD samples examined). Fewer LBs were stained by the α-synuclein antibodies in the substantia nigra (2 of 15 FAD samples examined; Figure 1D ▶ ) and in the locus ceruleus (1 of 12 FAD samples examined). No α-synuclein aggregates were seen in the cerebellum of any of the FAD cases, and, aside from brain, there were no α-synuclein-stained abnormalities in any of the other organs from the one FAD case studied here. Overall, 22% of the FAD cases contained LBs in at least one brain sample.
In the FAD cases, α-synuclein-positive dystrophic processes (so-called Lewy neurites, Figure 1c ▶ ) were seen occasionally in areas with LBs, including the amygdala, periamygdaloid cortex, cingulate gyrus, and substantia nigra. They also were observed occasionally in the CA2-3 region of the hippocampus, but these neurites were less prominent than those described in DLB and LBVAD 7-10 as well as those seen here in control DLB brains. Significantly, no LBs, dystrophic neurites, or other lesions were detected by antibodies to either β- or γ-synuclein in any of the FAD or control cases studied here. Finally, the ApoE genotype did not exert an obvious influence on the presence or distribution of α-synuclein-positive LBs or Lewy neurites in these FAD cases. For example, in the four FAD cases with the most abundant α-synuclein-stained LBs, three had an ApoE-ɛ3/3 genotype, and the other case had an ApoE-ɛ3/4 genotype.
The immuno-EM studies were performed to determine the ultrastructural localization of α-synuclein immunoreactivity in the LBs of the FAD brain, and these experiments showed that α-synuclein immunoreactivity was localized primarily to the filaments in LBs, although some α-synuclein staining also was associated with the amorphous and granular material interspersed between these filaments (Figure 1G) ▶ . Thus, as suggested by previous studies, 9 these findings imply that α-synuclein is a major component of LB filaments.
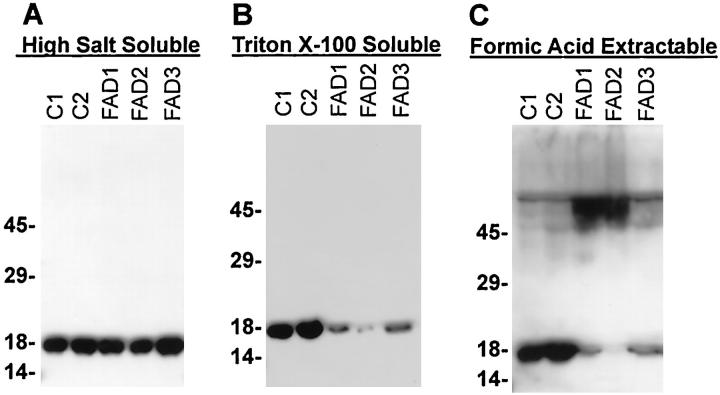
Although limited amounts of unfixed, frozen FAD amygdala suitable for biochemical studies were available, the Western blot analyses performed on α-synuclein-enriched extracts of the amygdala from three FAD and two normal control brains revealed approximately equal amounts of α-synuclein in the high-salt-soluble fraction (Figure 2A) ▶ . In formic-acid-extractable extracts, high molecular mass α-synuclein aggregates were detected in Western blots of the two FAD amygdala samples (FAD1 and FAD2, Figure 2C ▶ ) where α-synuclein-containing LBs were present. The FAD case without LBs (FAD3) and control brains lacked high molecular weight α-synuclein aggregates (Figure 2C) ▶ . The reduction in monomeric α-synuclein in formic-acid-soluble extracts from the three FAD cases may reflect a reduction in membrane-bound α-synuclein, as a reduction was also observed in the high-salt-insoluble, Triton X-100-soluble fractions (Figure 2B) ▶ .
Figure 2.
Western blot analysis performed with MAb LB509 demonstrated high molecular mass aggregates of α-synuclein in the amygdala from FAD patients with PS-1 mutations. Amygdalas from control and FAD patients were fractionated as described in Materials and Methods. A: α-Synuclein in the high-salt-soluble fractions from the amygdala of three FAD cases (FAD1-3) compared with the amygdala from two control cases (C1-2). B: α-Synuclein in the high-salt-insoluble, Triton X-100-soluble fractions. C: α-Synuclein in formic-acid-extractable fractions. Five microliters of high-salt-insoluble, Triton X-100-soluble extracts or 10 μl of formic-acid-extractable extracts was loaded in each lane, respectively. The Western blots developed from high-salt-soluble and Triton X-100-soluble extracts were exposed to film for the same length of time, whereas the blot developed from formic-acid-extractable samples was exposed approximately five times longer.
Discussion
This is the first study to determine whether α-synuclein is a component of LBs in the FAD brain, and we show here that antibodies to α- but not β- or γ-synuclein label LBs in at least 22% of a large series (n = 74) of FAD brains. Remarkably, of those FAD brains from which the amygdala was available for analysis, >60% showed α-synuclein-immunoreactive LBs. Thus, the prevalence of LBs in the brains of FAD patients with a known mutation in the PS-1, PS-2, or APP genes may be far higher than that suggested by previous studies. Furthermore, the antibodies to α-synuclein-stained LBs in many of the brain regions in which they have been shown to exist in DLB and LBVAD, including the deeper layers of the parahippocampal gyrus and cingulate cortex. However, classical appearing α-synuclein-positive nigral LBs and Lewy neurites (including those typically seen in CA2-3) were less common in these FAD brains compared with the brains of sporadic DLB and LBVAD patients. 7-10 Although there are few electron microscopic studies of LBs in FAD brains, our immuno-EM data indicate that the filaments in these LBs are decorated by antibodies to α-synuclein and that these LBs closely resemble those in PD, DLB, and LBVAD. In addition, just like the DLB brain, our quantitative Western blot analyses demonstrated that α-synuclein in LB-rich regions of FAD brains aggregates into high molecular weight species and is less soluble that α-synuclein in normal brains. Finally, α-synuclein-stained lesions were not present in any tissues outside of the brain in one of the FAD patients studied here, and the antibodies to β- or γ-synuclein did not stain LBs or other lesions in the FAD brains.
The biological trigger for focal alterations in α-synuclein expression and/or solubility in FAD is unknown. However, our observations are important because they extend the disorders in which α-synuclein aggregates into LBs to include degenerative diseases with mutations unrelated to those causing familial PD. Thus, genetic mutations involving genes other than the α-synuclein gene may result in a sequence of events that locally influence α-synuclein and induce LB formation.
The mechanism by which α-synuclein forms into LBs is unknown. However, alterations in the expression, metabolism, or biochemical properties of α-synuclein may lead to a reduction in solubility followed by its incorporation into LB filaments. Our data showing an increase in the pool of insoluble α-synuclein in the FAD amygdala are consistent with data from other studies showing a diminution in the solubility of α-synuclein recovered from neurodegenerative disease brains with abundant LBs relative to normal brains. 9 Whether or not epigenetic factors play a role in LB formation in the brains of FAD patients is not clear at this time, but if our data on the prevalence of α-synuclein-positive LBs in the limited number of amygdala samples from FAD patients accurately reflects the prevalence of LBs in the brains of FAD patients with mutations in PS-1, PS-2, or APP genes, then it is reasonable to infer that these mutations indeed play a role in the formation of LBs from abnormal filaments composed of α-synuclein.
Our data confirm the findings of others showing that α-synuclein is a sensitive and specific protein marker of LBs, 7-10 although antibodies to α-synuclein detected LBs in the brains of all but two FAD patients in whom LBs had been identified earlier using anti-ubiquitin antibodies and histochemical stains. 30 The reason LBs were not labeled by the antibodies to α-synuclein in these two FAD brains is not clear, but it is possible that this reflects the low abundance of LBs in these brains or that the inclusions identified previously were ubiquitin-positive globose NFTs, which can be difficult to distinguish from ubiquitin-positive LBs. Indeed, the latter problem undoubtedly accounts for the fact that LBs are not recognized as common neuropathological lesions in the brains of FAD patients due to mutations in PS-1, PS-2, or APP genes, although LBs have been described by a number of authors in AD cases that result from genetic abnormalities, including Down’s syndrome. 11-14 Thus, our data suggest that pathogenic mutations other than those that cause familial PD by affecting the α-synuclein gene may induce LB formation. Although there is no direct evidence linking mutations in PS-1, PS-2, or APP genes to perturbations in the metabolism of wild-type α-synuclein, our data suggest that it may be timely now to investigate potential interactions of the mutant gene products that result from these well known FAD mutations similar to several previous studies of other relevant protein interactions. 31, 32 For example, although co-localization of NFTs and LBs has been described previously in the brains of patients with sporadic DLB and PD, 33 the present studies suggest that genetic factors may play a role in the co-occurrence of these two different inclusions in the same neuron. Thus, studies of the sequence of events that underlie the formation of LBs, NFTs, and Aβ plaques may clarify the role of genetic and epigenetic factors in the pathogenesis of these lesions.
Acknowledgments
We acknowledge Drs. R. Martins, J. Krill, J. Arango, C. Van Broeckhoven, P. Cras, M. Haltia, A. Jorgensen, R. Ellison, C. Hulette, B. Hyman, and S. Sato for contributing cases. We also acknowledge the assistance of D. Lavalla and S. Chiu and our many colleagues in our research programs. A number of other investigators fostered this research by recruiting and donating precious PS-1 and APP mutation sections. Lastly, we thank the families of patients studied here for their generosity and commitment to this research because without them this research would not be possible.
Footnotes
Address reprint requests to Dr. Carol F. Lippa, Department of Neurology, Allegheny University of the Health Sciences, 3300 Henry Avenue, Philadelphia, PA 19129. E-mail: lippa@auhs.edu.
Supported by the National Institute on Aging of the National Institutes of Health (AG13623, AG09215, AG10124, AG05136, AG10133, and AG06781) and the Robert Potamkin Fund.
References
- 1.Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH: Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2–3 neurites specific to DLBD. Neurology 1991, 41:1402-1409 [DOI] [PubMed] [Google Scholar]
- 2.Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK: Senile dementia of Lewy body type: a clinically and neuropathologically distinct type of Lewy body dementia in the elderly. J Neurol Sci 1990, 95:119-139 [DOI] [PubMed] [Google Scholar]
- 3.Lennox G, Lowe R, Morrell R, Landon M, Mayer RJ: Anti-ubiquitin immunochemistry is more sensitive than conventional techniques in the detection of diffuse Lewy body disease. J Neurol Neurosurg Psychiatry 1989, 52:67-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M, Rice V, Butters N, Alford M: The Lewy body variant of Alzheimer’s disease: a clinical and pathological entity. Neurology 1990, 40:1-8 [DOI] [PubMed] [Google Scholar]
- 5.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276:2045-2047 [DOI] [PubMed] [Google Scholar]
- 6.Krüeger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O: Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature Genet 1998, 18:106-108 [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M: α-synuclein in Lewy bodies. Nature 1997, 388:839-840 [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H: NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson’s disease. Neurosci Lett 1997, 239:45-48 [DOI] [PubMed] [Google Scholar]
- 9.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM-Y, Trojanowski JQ, Iwatsubo T: Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 1998, 152:879-884 [PMC free article] [PubMed] [Google Scholar]
- 10.Irrizary MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT: Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain α-synuclein immunoreactivity. J Neuropathol Exp Neurol 1998, 57:334-337 [DOI] [PubMed] [Google Scholar]
- 11.Raghavan R, Khin-Nu C, Brown A, Irving D, Ince PG, Day, Tyrer SP, Perry RH: Detection of Lewy bodies in trisomy 21 (Down’s syndrome). Can J Neurol Sci 1993, 20:48–51 [DOI] [PubMed]
- 12.Bodhireddy S, Dickson D, Mattice L, Weidenheim KM: A case of Down’s syndrome with diffuse Lewy body disease. Neurology 1994, 44:159-161 [DOI] [PubMed] [Google Scholar]
- 13.Lippa CF, Smith TW, Nee L, Robitaille Y, Crain B, Dickson D, Pulaski-Salo D, Pollen DA: Familial Alzheimer’s disease and cortical Lewy bodies. Is there a genetic susceptibility factor? Dementia 1995, 6:97-103 [DOI] [PubMed] [Google Scholar]
- 14.Hulette CM, Rosenberg CK, Pericak-Vance MA, Gilbert JR, Gaskell PC, Roses AD: Lewy body and Alzheimer pathology in families with the amyloid precursor protein (APP717) gene mutation. Neurology 1998, 49:A232. [DOI] [PubMed] [Google Scholar]
- 15.Goudsmit J, White BJ, Weitkamp LR, Keats BJB, Morrow CH, Gajdusek DC: Familial Alzheimer’s disease in two kindreds of the same geographic and ethnic origin. J Neurol Sci 1981, 49:79-89 [DOI] [PubMed] [Google Scholar]
- 16.Struble RJ, Polinsky RJ, Hedreen JC, Nee LE, Frommelt P, Feldman RG, Price DL: Hippocampal lesions in dominantly inherited Alzheimer’s disease. J Neuropathol Exp Neurol 1991, 50:82-94 [DOI] [PubMed] [Google Scholar]
- 17.Foncin JF, Salmon D, Supino-Viterbo V, Feldman RG, Macchi G, Mariotti P, Scoppeta C, Caruso G, Bruni AC: Demence presenile d’Alzheimer transmise’dans une famille etendue. Rev Neurol (Paris) 1985, 141:194-202 [PubMed] [Google Scholar]
- 18.Nee LE, Polinsky RJ, Eldridge R, Weingartner J, Smallberg S, Ebert M: A family with histologically confirmed Alzheimer’s disease. Arch Neurol 1983, 40:203-208 [DOI] [PubMed] [Google Scholar]
- 19.Murrell J, Farlow MR, Ghetti B, Benson MD: A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991, 254:97-99 [DOI] [PubMed] [Google Scholar]
- 20.Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KA, Weber JL, Bird TD, Schellenberg GD: A familial Alzheimer’s disease focus on chromosome 1. Science 1995, 269:970-973 [DOI] [PubMed] [Google Scholar]
- 21.Nochlin D, Bird TD, Nemens EJ, Ball MJ, Sumi SM: Amyloid angiopathy in a Volga German family with Alzheimer’s disease and a PS-2 mutation (N1411). Ann Neurol 1998, 43:131-135 [DOI] [PubMed] [Google Scholar]
- 22.: The National Institute on Aging, Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s disease: Consensus recommendations for the postmortem diagnosis of Alzheimer disease. Neurobiol Aging 1997, 18:S1-S3 [PubMed] [Google Scholar]
- 23.Braak H, Braak E: Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995, 16:271-284 [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quin NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, de Vos RAI, Wilcock GK, Jellinger KA, Perry RH: Consensus guidelines for the clinical pathological diagnosis of dementia with Lewy bodies: report of the Consortium on Dementia with Lewy Bodies International Workshop. Neurology 1996, 47:1113-1124 [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman DA, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34:939-944 [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE: Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res 1997, 57:759-764 [PubMed] [Google Scholar]
- 27.Jakes R, Spillantini MG, Goedert M: Identification of two distinct synucleins from human brain. FEBS Lett 1994, 345:27-32 [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Key ME, Kalra KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 29.Tu PH, Robinson KA, de Snoo F, Eyer J, Peterson A, Lee VM, Trojanowski JQ: Selective degeneration of Purkinje cells with Lewy body-like inclusions in aged NFHLACZ transgenic mice. J Neurosci 1997, 17:1064-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lantos P, Luthert P, Hanger D, Anderton BH, Mullan M, Rossor M: Familial Alzheimer’s disease with the amyloid precursor protein position 717 mutation and sporadic Alzheimer’s disease have the same skeletal pathology. Neurosci Lett 1992, 137:221-224 [DOI] [PubMed] [Google Scholar]
- 31.Selkoe DJ: Alzheimer’s disease: genotypes, phenotype, and treatment. Science 1997, 275:630-631 [DOI] [PubMed] [Google Scholar]
- 32.DeStrooper B, Saftig P, Craessaerts K, Vanderstichele, G Guhde, Annaert W, Von Figura, Van Lauven F: Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 1998, 391:387–390 [DOI] [PubMed]
- 33.Schmidt ML, Martin JA, Lee VM-Y, Trojanowski JQ: Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons in Alzheimer’s disease and Lewy body disorders. Acta Neuropathol (Berl) 1996, 91:475-481 [DOI] [PubMed] [Google Scholar]