Abstract
This study investigated the presence of platelet-activating factor (PAF) in the lipid extracts of 18 primary breast carcinomas and 20 control breast tissues. The amount of PAF detected in breast carcinomas was significantly higher than in controls. The mass spectrometric analysis of PAF-bioactive lipid extract from breast carcinomas showed the presence of several molecular species of PAF, including C16-alkylPAF, C18-lysophosphatidylcholine (LPC), C16-LPC, lyso-PAF, and C16-acylPAF. The amount of bioactive PAF extracted from breast specimens significantly correlated with tumor vascularization revealed by the number of CD34- and CD31-positive cells. As C16-alkylPAF was previously shown to induce angiogenesis in vivo, we evaluated whether the thin layer chromatography-purified lipid extracts of breast specimens elicited neoangiogenesis in a murine model of subcutaneous Matrigel injection. The lipid extracts from specimens of breast carcinoma containing high levels of PAF bioactivity, but not from breast carcinomas containing low levels of PAF bioactivity or from normal breast tissue, induced a significant angiogenic response. This angiogenic response was significantly inhibited by the PAF receptor antagonist WEB 2170. T47D and MCF7 breast cancer cell lines, but not an immortalized nontumor breast cell line (MCF10), released PAF in the culture medium. A significant in vivo neoangiogenic response, inhibited by WEB 2170, was elicited by T47D and MCF7 but not by MCF10 culture medium. These results indicate that an increased concentration of PAF is present in tumors with high microvessel density and that PAF may account for the neoangiogenic activity induced in mice by the lipid extracts obtained from breast cancer. A contribution of PAF in the neovascularization of human breast cancer is suggested.
Considerable experimental evidence indicates that formation of new blood vessels is required for tumor growth. 1,2 Moreover, new vessels penetrating into the tumor are frequent sites for entry of tumor cells into the circulation and for formation of metastasis. 2-4 The neoangiogenesis may also be required for the expansion of the metastatic colony. 5-9 It is controversial whether the neoangiogenesis of the primary breast cancer is an independent prognostic marker or not. 10-17 However, several studies have suggested that the growth and the metastatic dissemination of human breast cancer correlates with the process of angiogenesis. 9,18-20 Soluble mediators produced by tumor and inflammatory cells have been involved in neoangiogenesis. 4,21 These include polypeptide mediators, such as cytokines and growth factors, nitric oxide, and lipid mediators. 21-24 In human breast cancer, many angiogenic factors have been related to estrogen regulation of growth and to tumor vascularization. 25-36 Recent studies link the platelet-activating factor (PAF), a phospholipid mediator of inflammation, 37 to the biological activities of certain polypeptide mediators. 38 It has been found that the angiogenesis induced by tumor necrosis factor (TNF) and hepatocyte growth factor (HGF) is partially due to biosynthesis of PAF. 39,40 PAF, in turn, directly stimulates in vitro migration of endothelial cells and promotes in vivo angiogenesis. 41,42 PAF, which is produced by a broad range of cells, including neutrophils, macrophages, and endothelial cells (reviewed in Refs. 37 and 43 ), acts through a specific receptor belonging to the family of seven-domain membrane-spanning receptors. 44 It has been reported that PAF triggers diverse and potent biological properties relevant for the development of inflammatory reaction, embryogenesis, and cell differentiation. 37,43 The presence of PAF was detected in human breast carcinomas but not in nontumor breast tissue. 45 Recently, the production of PAF has been correlated with the formation of pulmonary metastasis in a murine model of melanoma. 46 In addition, PAF was shown to mediate the metastasis-promoting activities of TNF-α and interleukin (IL)-1α. 46 Preliminary studies indicate an involvement of PAF in inflammatory neoangiogenesis occurring in humans. 47 In contrast, the potential role of this mediator in tumor angiogenesis has not yet been explored. The aim of the present study was to evaluate whether the production of PAF within human breast cancer correlates with the extent of neovascularization of the tumor and whether tumor-extracted PAF induces neoangiogenesis in an in vivo murine model.
Materials and Methods
Tissue Collection
Specimens from 18 patients with primary invasive breast carcinoma (11 ductal, 5 lobular, and 2 mixed ductal/lobular carcinomas; age range, 45 to 85 years; Table 1 ▶ ) and from 20 controls (7 normal breast tissues and 13 benign breast lesions such as fibroadenomas, fibrocystic changes, and sclerosing adenosis; age range, 42 to 75 years) were obtained after surgery. Adjacent cross sections of specimens were processed for PAF extraction and histological analysis.
Table 1.
Clinical and Immunohistochemical Characteristics of Breast Cancers
| Patients | Type | T | N | G | ER (%) | PR (%) | K67 (%) | Cat.S (%) | Cat.N (%) | p53 (%) | cErb (%) | PAF (pg/mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DIC | 1c | 0 | 1 | 95 | 90 | 14 | 5 | 5 | 0 | 50 | 1 |
| 2 | DIC | 1c | 1 | 2 | 95 | 70 | 45 | 15 | 60 | 5 | 0 | 71 |
| 3 | LIC | 2 | 0 | 3 | 0 | 0 | 43 | 8 | 0 | 90 | 80 | 1.5 |
| 4 | DIC | 2 | 0 | 3 | 80 | 60 | 35 | 5 | 0 | 0 | 0 | 0.9 |
| 5 | DIC+ LIC | 4 | 1 | 2 | 60 | 0 | 50 | 5 | 30 | 10 | 0 | 27 |
| 6 | DIC+ LIC | 1c | 0 | 2 | 90 | 50 | 25 | 0 | 6 | 0 | 65 | 57 |
| 7 | DIC | 3 | 0 | 2 | 90 | 90 | 33 | 35 | 60 | 3 | 80 | 0 |
| 8 | DIC | 1c | 0 | 1 | 0 | 0 | 48 | 0 | 40 | 60 | 90 | 13.3 |
| 9 | DIC | 1c | 0 | 1 | 75 | 80 | 19 | 0 | 0 | 0 | 0 | 0.2 |
| 10 | LIC | 1c | 0 | 1 | 90 | 30 | 10 | 0 | 0 | 0 | 0 | 0.4 |
| 11 | DIC | 2 | 0 | 1 | 50 | 70 | 12 | 0 | 0 | 0 | 0 | 137 |
| 12 | DIC | 2 | 0 | 1 | 95 | 60 | 20 | 0 | 0 | 0 | 0 | 40 |
| 13 | DIC | 1c | 1 | 1 | 70 | 90 | 6 | 0 | 0 | 0 | 0 | 0.1 |
| 14 | DIC | 2 | 0 | 3 | 0 | 0 | 60 | 20 | 20 | 90 | 0 | 0.2 |
| 15 | DIC | 1c | 0 | 1 | 0 | 0 | 19 | 20 | 80 | 2 | 80 | 16 |
| 16 | LIC | 2 | 1 | 3 | 0 | 0 | 60 | 10 | 0 | 0 | 0 | 0 |
| 17 | DIC | 1c | 1 | 1 | 90 | 15 | 9 | 0 | 0 | 0 | 75 | 34 |
| 18 | LIC | 1c | 0 | 3 | 90 | 90 | 31 | 0 | 0 | 5 | 80 | 0.4 |
DIC, ductal infiltrating carcinoma; LIC, lobular infiltrating carcinoma; ER, estrogen receptor; PR, progesteron receptor; Cat., cathepsin. PAF extracted from breast cancers was expressed as pg/mg dry tissue.
Histological Studies
Samples were fixed in buffered formalin and paraffin embedded. Sections were stained with hematoxylin and eosin (H&E) for histological diagnosis. Representative sections of the tumors including the infiltrating edges were selected and processed for immunohistochemistry. For the purpose of heat-induced antigen retrieval, sections were pretreated in a pressure cooker in a 10 nmol/L citrate buffer (pH 6.0) solution. A standard avidin-biotin-peroxidase complex procedure was applied. The assessment of vascularization was performed using anti-CD31 monoclonal antibody (MAb; diluted 1:30; Dako, Glostrup, Denmark) and anti-CD34 MAb (diluted 1:50; Novocastra Laboratories, New Castle upon Tyne, UK). After staining, sections were scanned at low power for hot spots of angiogenesis. Microvessels were evaluated on three ×200 fields. Any endothelial cell or cluster of endothelial cells positive for CD31 or CD34 was counted. Cathepsin D expression was evaluated using MAb M1G8 (diluted 1:1; CIS BIO International, Gif Sur Yvette, France). The percentage of the overall staining contributed solely by carcinoma cells and the percentage contributed only by stromal staining were scored. Hormonal receptor expression was evaluated using estrogen receptor MAb ER-1D5 (diluted 1:50; Dako) and progesterone receptor MAb PGR (diluted 1:50; Abbott Diagnostic, Wiesbaden-Delkenheim, Germany). p53 protein expression was studied using the MAb DO-7 (diluted 1:100; Biogenex, San Ramon, CA), and c-erbB-2 oncoprotein was demonstrated with a MAb HER-2/neu (diluted 1:100; Pabish, Pero, Italy). Only cell membrane immunoreactivity was regarded as specific for c-erbB-2 overexpression. Proliferation rate was assessed using a Ki67-related MAb (MIB1; diluted 1:10; Immunotech, Marseille, France).
Breast Cell Lines
The T-47D and MCF7 breast adenocarcinoma cell lines were obtained from American Type Culture Collection (Manassas, VA) and nontumor mammary gland MCF10A cell line was obtained from the Michigan Cancer Foundation (Detroit, MI). Cells were cultured in RPMI containing 10% fetal calf serum (Sigma Chemical Co, St. Louis, MO). Cells grown at confluence in 35-mm Petri dishes were washed twice and cultured overnight in RPMI containing 0.25% bovine serum albumin (BSA fraction V; Sigma). PAF was extracted from the cell supernatant as previously described. 48
Extraction and Quantitation of PAF
Lipids were extracted from breast specimen homogenates or from culture medium by chloroform/methanol/water acidified to pH 3.0 to 3.5 with formic acid as previously described. 47 PAF was quantified by bioassay on washed rabbit platelets after purification from the lipid extract by thin layer chromatography (TLC; silica gel 60, F254, Merck; solvent system: chloroform/methanol/water, 65:35:6, v:v) and high-pressure liquid chromatography (HPLC; μPorasil Millipore Chromatographic Division, Waters, Bedford, MA; mobile phase: chloroform/methanol/water, 60:55:5, v:v; flow rate, 1 ml/minute) as previously described. 48,49 The recovery of radioactive standards (Du Pont-NEN, Brussels, Belgium), submitted to the same procedures of extraction and TLC and HPLC purification of biological samples was, respectively, 96 to 98% for 1-O-[3H]-alkyl-PAF C16:O, 96 to 98% for 1-O-[3H]-alkyl-PAF C18:O, and 79 to 82% for [14C]-acyl-PAF C16:O. The specificity of platelet aggregation was inferred from the inhibitory effect of 3 μmol/L WEB 2170, a PAF receptor antagonist (Boehringer, Ingelheim, Germany). 50 PAF bioactivity was not inhibited by phospholipase A1 (Sigma), thus suggesting that it is related to alkyl-PAF rather than to acyl-PAF, which is known to be more than 1000 times less active than alkyl species of PAF. 37 The bioactive material was further characterized as PAF on the basis of TLC and HPLC behavior and of physicochemical characteristics, 45,49 such as inactivation by strong bases and phospholipase A2 (Sigma) but resistance to phospholipase A1 and acidic treatment. After TLC and HPLC purification, PAF-bioactive material was also analyzed by a recently developed technique based on HPLC (normal phase silica column μPorasil, 250 × 4.6 mm internal diameter (Millipore Waters), eluted under isocratic conditions at 1.0 ml/minute, with a mobile phase composed of chloroform/methanol/water, 60:55:5, v:v) tandem mass spectrometry (HPLC-MS/MS). 51 To reduce the mobile phase flow rate to a level compatible with the MS system, post-column splitting was performed by connecting a silica capillary of adequate length to the splitting port of the MS interface. The samples were injected dissolved in the mobile phase using an injection volume of 250 ml and a 200-ml sample loop. Mass spectrometric analysis was performed on a Perkin-Elmer-Sciex (Thornhill, Canada) API III-Plus triple quadrupole mass spectrometer, equipped with an atmospheric pressure articulated ion spray source. High-purity nitrogen served both as the nebulizer gas (operative pressure was 0.5 MPa) and curtain gas (flow rate was 0.8 L/minute). Argon was used as the target gas for the MS/MS experiments, at a collision gas thickness of 3 × 10 15 atoms/cm2. The ion spray needle voltage was set at 5 kV, the orifice voltage at 50 V, and the MS collision energy at 25 V, which were previously shown optimal conditions for these analyses. 51 The quantitative analysis was performed in multiple reaction monitoring (MRM). Parent ion spectra, positive mode, were obtained from daughter ions with mass-to-charge ratio (m/z) 184 corresponding to phosphocholine fragment; the scanning range was m/z 100 to 600. In the MRM analysis, acquired in positive mode, the study of different PAF molecular species was done using the following reactions (parent ions→daughter ions): 552→184 (C18-alkylPAF), 524→184 (C16-alkylPAF), 524→184 (C18-lysophosphatidylcholine (LPC)), 496→184 (C16-LPC, lyso-PAF), 538→184 (C16-acylPAF), 566→184 (C18-acylPAF), and 594→210 (CV3988, used as internal standard). CV3988 was kindly provided by Takeda (Osaka, Japan). All other standards were purchased from Sigma.
Murine Angiogenesis Assay
Female C57 mice at 6 to 8 weeks of age were used. Angiogenesis was assayed as growth of blood vessels from subcutaneous tissue into a solid gel of basement membrane (Matrigel, Becton Dickinson Labware, Bedford, MA) containing heparin (64 U/ml; Sigma) and the test sample. 52 Matrigel (8 mg/ml), in liquid form at 4°C, was mixed with the experimental substances and injected (0.5 ml) into the abdominal subcutaneous tissue of mice, along the peritoneal midline. The Matrigel used was extracted according to the procedure described by Taub et al, 53 which has been previously shown to efficiently deplete Matrigel of basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor I, and platelet-derived growth factor. 54,55
The angiogenic effect of 25 μl of TLC-purified lipid extract from breast tumor, control breast, or culture media from breast cell lines was evaluated in the absence or in the presence of WEB 2170. WEB 2170 was included in the Matrigel plug (final concentration, 250 ng/ml) and injected intraperitoneally (i.p.; 10 mg/kg) 30 minutes before the subcutaneous injection and daily for 6 days. In preliminary experiments, these concentrations of WEB 2170 were shown to inhibit angiogenesis induced by 12.5 ng of synthetic PAF. 39,47 Moreover, the angiogenic effect of 5 ng of synthetic C16-alkylPAF or C16-acylPAF (Bachem Feinchemikalien, Bubendorf, Switzerland) was assessed. After 6 days, mice were killed and gels were recovered and processed for light or for immunofluorescence microscopy, performed as previously described. 39,47 For immunofluorescence studies, rabbit anti-human von Willebrand factor (vWF) antibody (Sigma), anti-mouse T-cell serum MAb, and anti-Mac-1 FITC-conjugated MAb (Cedarlane, Ontario, Canada) were used. Vessel area and the total Matrigel area were planimetrically assessed from stained sections. 56 We considered vessels only those structures possessing a patent lumen and containing red blood cells. Results are expressed as percentage ± SE of the vessel area to the total Matrigel area.
Results
PAF was extracted and purified from tissue specimens of 18 patients with primary invasive breast carcinoma (22.36 ± 8.50 pg of PAF/mg dry tissue; mean ± SE) and from 20 control specimens (1.96 ± 1.07 pg of PAF/mg dry tissue; mean ± SE). The amount of PAF detected in specimens of breast carcinomas was significantly higher (Student’s t-test; P < 0.05) than in controls (Figure 1A) ▶ .
Figure 1.
Quantitation of PAF and vessel density in breast tissues. A: The amount of PAF extracted and purified from 18 primary invasive breast carcinomas and 20 controls is expressed as picograms of PAF per milligram of dry tissue. The mean ± SE is indicated (*P = <0.05, t-test). B: Vessel density is expressed as number of endothelial cells or clusters of endothelial cells positive for CD31 (open bar) and CD34 (hatched bar) per microscopic field (see Materials and Methods). Data are expressed as mean ± SE. C: Linear regression analysis between CD31-positive cells and concentration of PAF in breast carcinomas. D: Linear regression analysis between CD34-positive cells and concentration of PAF in breast carcinomas.
The bioactivity detected by washed rabbit platelet aggregation and inhibited by WEB 2170, a specific PAF receptor antagonist, should be attributed to the 1-alkyl derivatives of PAF as the platelet aggregation bioassay is relatively insensitive to the acyl derivatives. 37 Moreover, PAF-bioactive material extracted and purified from primary invasive breast carcinoma was insensitive to treatment with phospholipase A1, which cleaves the acyl- but not the alkyl-PAF. 37,49 To evaluate the efficiency of phospholipase A1 treatment, three samples containing PAF-bioactive material were added with [14C]acyl-PAF before treatment with phospholipase A1. 57 The amount of [14C]acyl-PAF hydrolyzed (recovered as a free fatty acid) was 86 ± 2.75, whereas the biological activity was not significantly reduced (92 ± 3.01% recovered activity).
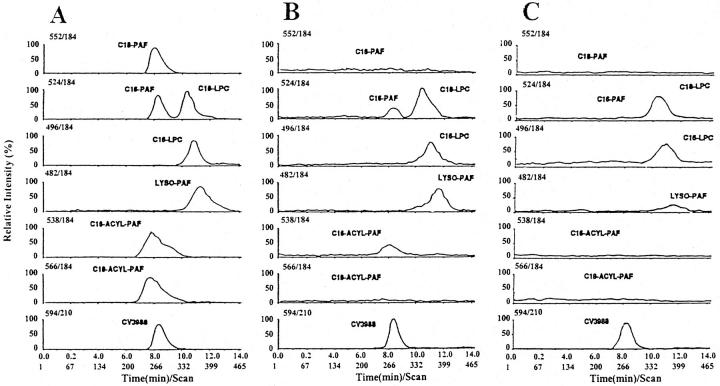
The lipid extracts of breast carcinomas and of controls were studied by HPLC-MS/MS. Figure 2A ▶ shows the representative HPLC-MS/MS chromatograms of a mixture of phospholipid standards containing C18-alkylPAF, C16-alkylPAF, C18-LPC, C16-LPC, lyso-PAF, C16-acylPAF, C18-acylPAF, and CV3988, obtained by MRM detection at m/z (parent→daughter) 552→184, 524→184, 524→184, 496→184, 482→184, 538→184, 566→184, and 594→210, respectively. Figure 2B ▶ shows the representative HPLC-MS/MS chromatograms of an individual sample of lipid extract from a breast carcinoma positive for PAF bioassay. Five samples were studied with similar results. The HPLC-MS/MS chromatographic analysis obtained by MRM of lipid extracts from breast carcinomas positive for PAF bioassay showed chromatographic peaks with MS/MS fragmentation and retention time corresponding to the standards C16-alkylPAF, C18-LPC, C16-LPC, lyso-PAF, and C16-acylPAF. CV3988 was used as internal standard (Figure 2B) ▶ . In breast carcinomas containing low levels of PAF bioactivity (<1.5 pg of PAF/mg of dry tissue; five individual samples) and in normal breast tissues (<0.25 pg of PAF/mg of dry tissue; five individual samples), the chromatographic peaks corresponding to C16-alkylPAF and C16-acylPAF were absent (Figure 2C) ▶ .
Figure 2.
Chromatographic traces by MRM detection at m/z (parent→daughter) 552→184, 524→184, 524→184, 496→184, 482→184, 538→184, 566→184, and 594→210 corresponding, respectively, to C18-alkylPAF, C16-alkylPAF, C18-LPC, C16-LPC, lyso-PAF, C16-acylPAF, C18-acylPAF, and CV3988, obtained by injection of a mixture of purified standards (24 ng each; A) or by injection of a lipid extract from a breast carcinoma positive for PAF-bioassay (B) or from a control breast tissue (C), spiked with 33 ng of CV 3988 as internal standard. Five individual samples for each group were submitted to HPLC-MS/MS chromatographic analysis with similar results.
Figure 1B ▶ shows the vascularization of breast cancer tissues as detected by CD31- and CD34-positive cells. The amount of PAF extracted from all breast specimens (tumors and controls) significantly correlated with the number of CD34-positive (r = 0.584; P = 0.003) and CD31-positive (r = 0.661; P = 0.001) cells. Moreover, Figure 1, C and D ▶ , shows the linear regression analysis performed between CD34- or CD31-positive cells and concentrations of PAF extracted from the specimens of breast carcinomas only. In contrast, no significant correlation was observed versus tumor markers such as Ki67 (r = −0.053; P = 0.834), p53 (r = −0.220; P = 0.380), c-erbB-2 (r = −0.306; P = 0.216), estrogen receptor (r = 0.344; P = 0.162), progesterone receptor (r = 0.288; P = 0.247), neoplastic cathepsin D (r = −0.034; P = 0.894), and stromal cathepsin D (r = −0.246; P = 0.310); nor was a correlation observed versus tumor grading. The histotypes of breast carcinomas containing high levels of PAF bioactivity were, in 6 of 11 patients, ductal carcinomas, in 2 of 2 patients, mixed ductal/lobular carcinomas, and, in none of 5 patients, lobular carcinomas (Table 1) ▶ .
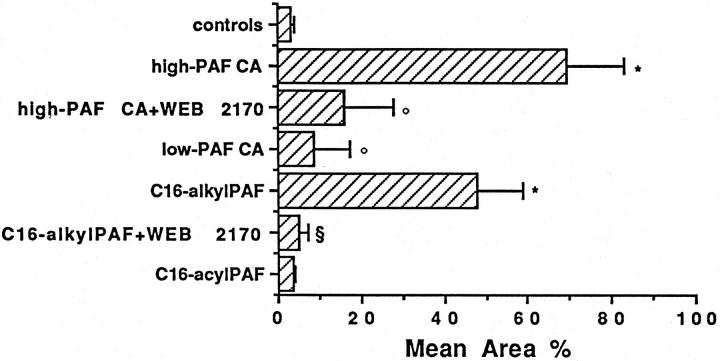
As PAF has been shown to induce angiogenesis in vivo, we evaluated whether the individual TLC-purified lipid extracts of breast specimens could elicit neoangiogenesis in a murine model of subcutaneous Matrigel injection. The lipid extracts from specimens of breast carcinoma containing high levels of PAF bioactivity (37.88 ± 9.4 pg of PAF/mg of dry tissue; mean ± SE; n = 6) induced a significant angiogenic response (Figures 3 and 4 ▶ ▶ , A and B). In contrast, the lipid extracts from specimens of breast carcinomas containing low levels of PAF bioactivity (0.57 ± 0.23 pg of PAF/mg of dry tissue; mean ± SE; n = 6, Figures 3 and 4D ▶ ▶ ) and from control specimens (0.24 ± 0.07 pg of PAF/mg of dry tissue; mean ± SE; n = 6, Figures 3 and 4E ▶ ▶ ) did not induce significant angiogenesis. Matrigel explanted from mice injected with the PAF extracted from specimens of breast carcinoma contained several Mac-1, esterase-positive monocytes but only scattered cells positive for pan-T lymphocyte markers. Infiltration of polymorphonuclear neutrophils was absent. The presence of endothelial cells in association with the vessels was confirmed by staining sections of the gel with anti-vWF antibodies (Figure 4C) ▶ . The treatment with the PAF receptor antagonist WEB 2170 significantly prevented neoangiogenesis induced by lipid extracted from specimens of breast carcinoma (Figures 3 and 4F) ▶ ▶ , thus suggesting a role for PAF as an angiogenic mediator. Control mice (n = 5) injected with vehicle alone and treated as above with WEB 2170 did not exhibit any cellular infiltration within Matrigel (data not shown). Synthetic C16-alkylPAF at concentrations compatible with that observed in the lipid extracts from specimens of breast carcinomas was able to induce angiogenesis (Figure 3) ▶ . The angiogenic response elicited by synthetic C16-alkylPAF was completely prevented by treatment of mice with WEB 2170. In contrast, synthetic C16-acylPAF induced a slight infiltration of Matrigel by vWF-positive endothelial cells without formation of canalized vessels.
Figure 3.
Quantitation of neovascularization performed on H&E-stained sections of Matrigel containing 10 U/ml heparin and 25 ng of lipid extract from six specimens of breast carcinoma containing high levels of PAF bioactivity, from six specimens of breast carcinoma containing low levels of PAF bioactivity, or from six control breast tissues or 5 ng of synthetic C16-alkylPAF or C16-acylPAF. Where indicated, WEB 2170 was included in the Matrigel plug (final concentration, 250 ng/ml) and injected i.p. (10 mg/kg) 30 minutes before the injection of Matrigel and daily for 6 days. Quantitation of neovascularization was performed on H&E-stained histological sections and results expressed as percentage ± SE of the vessel area to the total Matrigel area. ANOVA with Neuman Keuls multicomparison test was performed: controls versus high-PAF breast carcinomas, low-PAF breast carcinomas, C16-alkylPAF or C16-acylPAF (*P < 0.05); high-PAF breast carcinomas versus high-PAF breast carcinomas plus WEB 2170 or low-PAF breast carcinomas (°P < 0.05); C16-alkylPAF versus C16-alkylPAF plus WEB 2170 (§P < 0.05).
Figure 4.
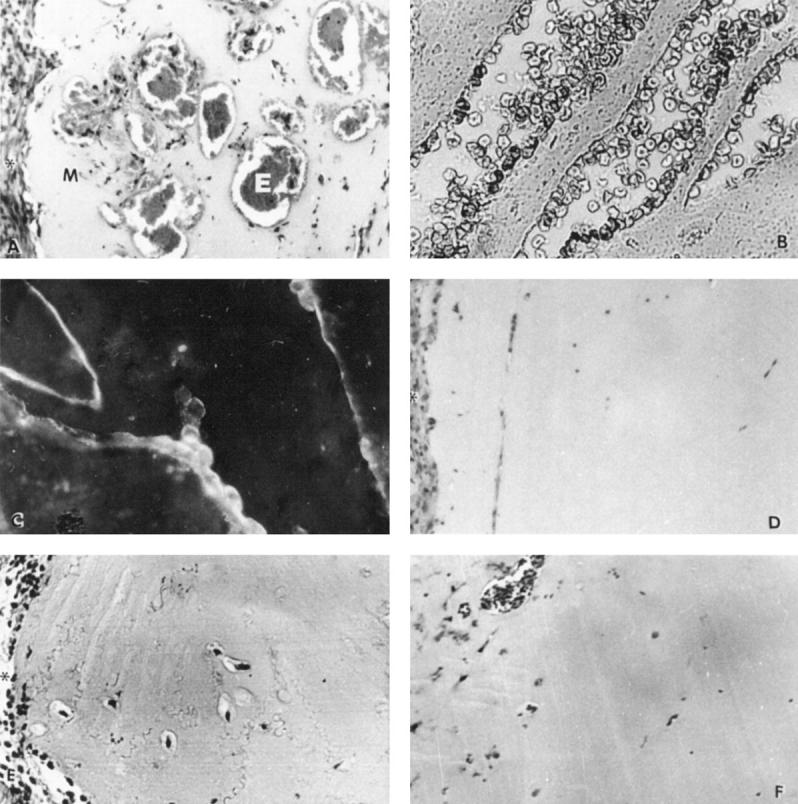
Representative histological analysis of Matrigel plugs excised from mice injected with 25 ng of lipid extract from a high-PAF breast carcinoma (A–C), a low-PAF breast carcinoma (D), or a control breast tissue (E) or from a high-PAF breast carcinoma in the presence of WEB 2170 (F). WEB 2170 was included in the Matrigel plug (final concentration, 250 ng/ml) and injected i.p. (10 mg/kg) 30 minutes before the injection of Matrigel and daily for 6 days. Canalized vessels and microanuerismatic structures containing erythrocytes (E) are seen in H&E-stained sections of Matrigel (M) containing the lipid extract from a high-PAF breast carcinoma (A and B). Asterisks indicate the subcutaneous tissue surrounding Matrigel. Endothelial cells, stained by indirect immunofluorescence for vWF, are underlying the lumen of a branched vessel in a section of Matrigel containing the lipid extract from a high-PAF breast carcinoma (C). D and E show the absence of canalized vessels in H&E-stained sections of Matrigel containing the lipid extract from a low-PAF breast carcinoma and from a control breast tissue. F shows the inhibitory effect of WEB 2170 on neovascularization of Matrigel containing the lipid extract from a high-PAF breast carcinoma. Magnification, ×250 (A, D, and F) and ×400 (B and C).
As shown in Table 2 ▶ , a significant amount of PAF was released in basal culture conditions by two breast cancer cell lines (T47D and MCF7) but not by an immortalized normal breast cell line (MCF10). When the culture medium of these cell lines was injected within Matrigel in mice, a significant neoangiogenic response was elicited by T47D and MCF7 cell lines but not by MCF10 or control medium. This angiogenic response was partially inhibited by the PAF receptor antagonist WEB 2170 (Table 2) ▶ .
Table 2.
PAF Release and in Vivo Angiogenic Properties of MCF-10A, MCF-7, and T-47D Breast Cell Lines
| Breast cell line | Release of PAF (ng/ml) | In vivo angiogenesis (mean area %) | Effect of WEB2170 (mean area %) |
|---|---|---|---|
| Control Medium | 0 | 3 ± 2 | ND |
| MCF-10A | 0 | 8 ± 2 | ND |
| MCF-7 | 42 ± 15 | 31 ± 5* | 11 ± 2† |
| T-47D | 110 ± 10 | 57 ± 13* | 9 ± 5‡ |
Basal PAF production was measured in the supernatant of two different breast cancer cell lines (MCF-7 and T-47D) and of a breast nontumor cell line (MCF-10A). Results are expressed as percentage ± SE of four different experiments. Lines 2 and 3 show neovascularization on Matrigel induced by 25 μl of the supernatants in the absence or in the presence of WEB 2170, a PAF receptor antagonist. Quantitation of neovascularization was performed on H&E-stained histological sections and results expressed as percentage ± SE of the vessel area to the total Matrigel area of four different experiments. ANOVA with Neuman Keuls multicomparison test was performed in the in vivo angiogenesis experiments: control medium versus MCF-10A, MCF-7, T-47D (*P < 0.05); T-47D + WEB 2170 versus T-47D or control medium (‡P < 0.05); MCF-7 + WEB 2170 versus MCF-7 or control medium (†P < 0.05). ND, not done.
Discussion
The results of the present study demonstrate that PAF is detectable in the lipid extracts of specimens of primary invasive breast carcinomas, that the amount of PAF correlates with the extent of intratumor angiogenesis, and that PAF extracted from breast carcinomas induces an angiogenic response when injected in mice.
Tumor vascularization is crucial for the pathogenesis of solid tumors. In the absence of local neovascular formation, tumors may not grow beyond 2 to 3 mm in diameter. 3 Therefore, it has been suggested that tumor neovascularization is a requirement for its growth. 3-5 Several studies have suggested that tumor angiogenesis in breast carcinoma is an independent prognostic factor. 10-15,17 The intratumor microvessel density has been correlated with tumor invasiveness and formation of metastases. The complex mechanisms involved in the formation of new vessels remain largely unknown. Certain tumors have been shown to produce factors that are directly angiogenic. Other tumors may depend upon vascularization induced by mediators produced from inflammatory cells. 21-24 Endothelial cells are the primary target for these mediators and are stimulated to degrade extracellular matrix, to migrate, and to initiate a capillary sprout. The sprout subsequently expands and assumes a tubular structure that, as result of endothelial cell proliferation, progresses in the extracellular matrix with development of loops and then of a functioning circulatory network. It has been shown that an imbalance in the expression of angiogenic and angiostatic factors occurs locally in tumors. 5,7 Studies on tumor angiogenesis have primarily focused on the role of angiogenic factors such as vascular endothelial growth factor (VEGF), TNF, IL-8, transforming growth factor-β, basic fibroblast growth factor, and tissue factor. 21-24 Several of these factors have been involved in the growth and in the vascular density of human breast carcinomas. 25-36 It has been recently shown that the endothelium in the breast cancers has a mitotic index 50-fold greater than that in nonmalignant tissues and that this proliferation is mainly present at the periphery of the tumor. 58 However, it has been suggested that different growth factors regulate the growth of tumor and of endothelium. 58
Recent studies indicate that PAF contributes to the angiogenesis induced by certain polypeptide mediators such as TNF and HGF. 39,40 Recently, we have shown the involvement of PAF in inflammatory neoangiogenesis in rheumatoid arthritis. 47 The results of the present study indicate that PAF may contribute to neoangiogenesis occurring in the breast carcinoma. Indeed, we found that different molecular species of PAF were detectable in the lipid extracts of breast carcinomas but not in normal breast. These included the alkyl-PAF C16, which is biologically active on platelets 37 and which is angiogenic in vivo. 41 The presence of PAF in the breast cancer tissues significantly correlated with the extent of tumor vascularization detected as number of CD31- and CD34-positive cells. Although the present study does not provide conclusive information on the origin of PAF detected in breast carcinomas, one can speculate that either infiltrating inflammatory cells or endothelial cells contribute to the synthesis of PAF within the tumor. 43,59 Moreover, we provide evidence that two breast cancer cell lines, but not a nontumor breast cell line, are able to release in basal condition bioactive PAF. The supernatants of these two breast cell lines were shown to induce an angiogenic response in vivo that was, at least in part, due to the presence of PAF, as inferred by the inhibitory effect of WEB 2170. These results are consistent with the previously reported ability of breast cancer cells to produce several mediators able to recruit inflammatory cells and/or to induce angiogenesis. 25-36 In addition, we observed that the lipid extracts of breast carcinomas containing high levels of PAF bioactivity, but not the lipid extracts of breast carcinomas containing low levels of PAF bioactivity or the lipid extract of normal breast, were angiogenic in vivo in mice. The observation that WEB 2170, a PAF receptor antagonist, significantly prevented angiogenesis induced by the lipid extracts of breast carcinomas suggests a key role of PAF in the mechanism of new vessel formation. The potential role of PAF in the progression of tumor disease has been recently addressed in an experimental model of melanoma. In this model, the production of PAF has been correlated with the formation of pulmonary metastasis. 46 In addition, PAF was shown to mediate the metastasis-promoting activities of TNF-α and IL-1α. 46 These observations further support the role of PAF as mediator of several biological activities of certain cytokines. 37,43 Angiogenic factors such as TNF-α, 60 IL-8, 61 or VEGF (unpublished observation) are all able to stimulate the synthesis of PAF. In this context, PAF may act as a mediator of cell-to-cell communication involved in the amplification of cytokine-triggered signal. PAF may act either as a chemoattractant for endothelial cells or as a mediator that amplifies and propagates the reaction by determining the production of heparin-binding growth factors. 41 Indeed, PAF induces the expression of several angiogenic factors and chemokines including basic and acidic fibroblast growth factor, placental growth factor, VEGF and its specific receptor flk-1, HGF, KC, and macrophage inflammatory protein 2. 42,62
In conclusion, the results of the present study suggest that PAF, which is present in the tumors with high microvessel density, may account, at least in part, for the neoangiogenic activity occurring in human breast cancer. The role of polypeptide mediators such as fibroblast growth factor and VEGF in the vascularization of this tumor has been well established, 25-29,36 and the contribution of several other cytokines and growth factors has been suggested. 30-36 In this context, a lipid mediator such as PAF, which can be produced by either inflammatory or cancer cells, may contribute to amplify the stimuli leading to the formation of new vessels.
Footnotes
Address reprint requests to Prof. Giovanni Camussi, Laboratorio di Immunopatologia, Istituto di Nefro-Urologia, Corso Dogliotti 14, 10126 Torino, Italy.
Supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) and MURST.
References
- 1.Gimbrone MA, Leapman SB, Cotran RS, Folkman J: Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 1972, 136:261-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimbrone MA, Cotran RS, Leapman SB, Folkman J: Tumor growth neovascularization: an experimental model using rabbit cornea. J Natl Cancer Inst 1974, 52:413-427 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Cotran R: Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol 1976, 16:207-215 [PubMed] [Google Scholar]
- 4.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nature Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 6.Hart IR, Saini A: Biology of tumour metastases. Lancet 1991, 339:1453-1457 [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Folkman J: Pattern and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1991, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 8.Weinstat-Saslow D, Steeg PS: Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB 1994, 8:401-407 [DOI] [PubMed] [Google Scholar]
- 9.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med 1991, 324:3-8 [DOI] [PubMed] [Google Scholar]
- 10.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Melis S, Gasparini G: Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1991, 84:1875-1887 [DOI] [PubMed] [Google Scholar]
- 11.Bosari S, Lee AKD, DeLellis RA, Wiley BD, Heatley GJ, Silverman ML: Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 1991, 23:755-761 [DOI] [PubMed] [Google Scholar]
- 12.Toi M, Kashitani J, Tominaga T: Tumor angiogenesis is an independent prognostic indicator in primary breast carcinoma. Int J Cancer 1993, 55:371-374 [DOI] [PubMed] [Google Scholar]
- 13.Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, Caffo O, Barbareschi M, Boracchi P, Marubini E, Pozza F: Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast-carcinoma. J Clin Oncol 1994, 12:454-466 [DOI] [PubMed] [Google Scholar]
- 14.Weidner N: Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995, 147:9-19 [PMC free article] [PubMed] [Google Scholar]
- 15.Heiman R, Ferguson D, Powers C, Recant WM, Weichselbaum RR, Hellman S: Angiogenesis as a predictor of long-term survival for patients with node-negative breast cancer. J Natl Cancer Inst 1996, 88:1764-1769 [DOI] [PubMed] [Google Scholar]
- 16.Van Hoef ME, Knox WF, Dhesi SS, Howell A, Schor AM: Assessment of tumor vascularity as a prognostic factor in lymph node negative invasive breast cancer. Eur J Cancer 1993, 29A:1141-1145 [DOI] [PubMed] [Google Scholar]
- 17.Fox SB: Tumour angiogenesis and prognosis. Histopathology 1997, 30:294-301 [DOI] [PubMed] [Google Scholar]
- 18.Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL: Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet 1992, 340:1120-1124 [DOI] [PubMed] [Google Scholar]
- 19.Gasparini G, Harris AL: Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol 1995, 13:765-782 [DOI] [PubMed] [Google Scholar]
- 20.Simpson JF, Ahn C, Battifora H, Esteban JM: Endothelial area as a prognostic indicator for invasive breast carcinoma. Cancer 1996, 77:2077-2085 [DOI] [PubMed] [Google Scholar]
- 21.Folkman J: Clinical applications of research on angiogenesis. N Engl J Med 1995, 26:1757-1763 [DOI] [PubMed] [Google Scholar]
- 22.Leek RD, Harris AL, Lewis CE: Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukocyte Biol 1994, 56:423-435 [DOI] [PubMed] [Google Scholar]
- 23.Blood CH, Zetter BR: Tumor interaction with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta 1990, 1032:89-118 [DOI] [PubMed] [Google Scholar]
- 24.Sunderskotter C, Steinbrink K, Goebeler M, Bhardway C, Sorg C: Macrophages and angiogenesis. J Leukocyte Biol 1994, 55:410-422 [DOI] [PubMed] [Google Scholar]
- 25.Dvorak HF, Lawrence FB, Detmar M, Dvorak A: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 26.Brown L, Berse B, Jakman R, Tognazzi K, Guidi A, Dvorak H, Senger D, Connolly J, Schnitt S: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995, 28:86-91 [DOI] [PubMed] [Google Scholar]
- 27.Toi M, Inada K, Suzuki H, Tominaga T: Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Br J Cancer Res Treat 1995, 36:193-204 [DOI] [PubMed] [Google Scholar]
- 28.Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP: Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res 1996, 56:2013-2016 [PubMed] [Google Scholar]
- 29.Anandappa SY, Winstanley JHR, Leinster S, Green B, Rudland PS, Barraclough R: Comparative expression of fibroblast growth factor mRNA in benign and malignant breast disease. Br J Cancer 1994, 69:772-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB: Evidence that transforming growth factor-β is a hormonally regulated negative growth factor in human breast cancer cells. Cell 1987, 48:417-428 [DOI] [PubMed] [Google Scholar]
- 31.Walker RA, Dearing SJ, Gallacher B: Relationship of transforming growth factor-β1 to extracellular matrices and stromal infiltrates in invasive breast carcinoma. Br J Cancer 1994, 69:1160-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, Shin S: Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res 1994, 54:1630-1636 [PubMed] [Google Scholar]
- 33.Pusztai L, Clover L, Cooper K, Starkey PM, Lewis CE, McGee JO: Expression of tumor necrosis factor and its receptors in carcinoma of the breast. Br J Cancer 1994, 70:289-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vrana JA, Stang MT, Grande JP, Getz MJ: Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor-β family. Cancer Res 1996, 56:5063-5070 [PubMed] [Google Scholar]
- 35.Contino J, Hair G, Kreutzer DL, Rickles FR: In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nature Med 1996, 2:209-218 [DOI] [PubMed] [Google Scholar]
- 36.Relf M, LeJeune S, Scott PAE, Fox S, Smith K, Leek R, Moghaddan A, Withouse R, Bicknell R, Harris AL: Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet derived endothelial cell growth factor, placental growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997, 57:963-969 [PubMed] [Google Scholar]
- 37.McManus LM, Woodard DS, Deavers SI, Pinckard SI: PAF molecular heterogeneity: pathobiological implications. Lab Invest 1993, 69:639-648 [PubMed] [Google Scholar]
- 38.Camussi G: Interactive effects of tumor necrosis factor and platelet activating factor in the pathogenesis of glomerular injury. Lab Invest 1994, 70:435-436 [PubMed] [Google Scholar]
- 39.Montrucchio G, Lupia E, Battaglia E, Passerini G, Bussolino F, Emanuelli G, Camussi G: Tumor necrosis factor induced angiogenesis depends on in situ platelet-activating factor byosynthesis. J Exp Med 1994, 180:377-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camussi G, Montrucchio G, Lupia E, Soldi R, Comoglio PM, Bussolino F: Angiogenesis induced in vivo by hepatocyte growth factor is mediated by platelet activating factor synthesis from macrophages. J Immunol 1997, 158:1302-1309 [PubMed] [Google Scholar]
- 41.Camussi G, Montrucchio G, Lupia E, DeMartino A, Perona L, Arese M, Vercellone A, Toniolo A, Bussolino F: Platelet-activating factor directly stimulates in vitro migration of endothelial cells and promotes in vivo angiogenesis by a heparin-dependent mechanism. J Immunol 1995, 154:6492-6501 [PubMed] [Google Scholar]
- 42.Bussolino F, Arese M, Montrucchio G, Barra L, Primo L, Benelli R, Sanavio F, Aglietta M, Ghigo D, Rola-Pleszczynski M, Bosia A, Albini A, Camussi G: Platelet activating factor produced in vitro by Kaposi’s sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest 1995, 96:940-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott SM, Zimmerman GA, McIntyre GA: Platelet-activating factor. J Biol Chem 1990, 268:17381-17384 [PubMed] [Google Scholar]
- 44.Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Myamoto T, Shimizu T: Cloning by functional expression of platelet activating factor receptor from guinea pig lung. Nature 1991, 349:342-346 [DOI] [PubMed] [Google Scholar]
- 45.Pitton C, Lanson M, Besson P, Fetissoff F, Lansac J, Benveniste J, Bougnoux P: Presence of PAF-acether in human breast carcinoma: relation to axillary lymph node metastasis. J Natl Cancer Inst 1989, 81:1298-1302 [DOI] [PubMed] [Google Scholar]
- 46.Im SY, Ko HM, Kim JW, Lee HK, Ha TY, Lee HB, Oh SJ, Bai S, Chung KC, Lee YB, Chun SB: Augmentation of tumor metastases by platelet-activating factor. Cancer Res 1996, 56:2662-2665 [PubMed] [Google Scholar]
- 47.Lupia E, Montrucchio G, Battaglia E, Modena V, Camussi G: Role of tumor necrosis factor-a and platelet activating factor in neoangiogenesis induced by sinovial fluids of patients with rheumatoid arthritis. Eur J Immunol 1996, 26:1690-1694 [DOI] [PubMed] [Google Scholar]
- 48.Camussi G, Aglietta M, Malavasi F, Tetta C, Piacibello W, Sanavio F, Bussolino F: The release of platelet activating factor from human endothelial cells in culture. J Immunol 1983, 131:2397-2403 [PubMed] [Google Scholar]
- 49.Bussolino F, Arese M, Silvestro L, Soldi R, Benfenati E, Sanavio F, Aglietta M, Bosia A, Camussi G: Involvement of a serine protease in the synthesis of platelet-activating factor by endothelial cells stimulated by tumor necrosis factor-α or interleukin-1α. Eur J Immunol 1994, 24:3131-3139 [DOI] [PubMed] [Google Scholar]
- 50.Heuer HO, Casals-Stenzel J, Muacevic G, Weber KH: Pharmacologic activity of bepafant (WEB2170), a new and selective tetrazepinoic antagonist of platelet-activating factor. J Pharmacol Exp Ther 1990, 225:962-968 [PubMed] [Google Scholar]
- 51.Rizea Savu S, Silvestro L, Sorgel F, Montrucchio G, Lupia E, Camussi G: Determination of 1-O-acyl-2-acetyl-sn-glyceryl-3-phosphorylcholine, platelet-activating factor and related phospholipids in biological samples by high-performance liquid chromatography by tandem mass spectrometry. J Chromatogr 1996, 628:35-42 [DOI] [PubMed] [Google Scholar]
- 52.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR: A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992, 67:519-528 [PubMed] [Google Scholar]
- 53.Taub M, Wang Y, Szczesny TM, Kleinman HK: Epidermal growth factor or transforming growth factor alpha is required for kidney tubulogenesis in Matrigel cultures in serum-free medium. Proc Natl Acad Sci USA 1990, 87:4002-4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH: Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 1992, 202:1-8 [DOI] [PubMed] [Google Scholar]
- 55.Santos OFP, Nigam SK: HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-β. Dev Biol 1993, 160:293-302 [DOI] [PubMed] [Google Scholar]
- 56.Kibbey MC, Grant DS, Klieinman HK: Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst 1992, 84:1633-1404 [DOI] [PubMed] [Google Scholar]
- 57.Triggiani M, Hubbard WC, Chilton FH: Synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine by an enriched preparation of the human lung mast cell. J Immunol 1990, 144:4773-4780 [PubMed] [Google Scholar]
- 58.Fox SB, Gatter KC, Bicknell R, Going JJ, Stanton P, Cooke TG, Harris AL: Relationship of endothelial-cell proliferation to tumor vascularity in human breast cancer. Cancer Res 1993, 53:4161-4163 [PubMed] [Google Scholar]
- 59.Barnes PJ, Page CP, Henson P: Platelet Activating Factor and Human Disease. 1989. Blackwell Scientific Publications, London
- 60.Camussi G, Bussolino F, Salvidio G, Baglioni C: Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med 1987, 166:1390-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bussolino F, Sironi M, Bocchietto E, Mantovani A: Synthesis of platelet-activating factor by polymorphonuclear neutrophils stimulated with interleukin-8. J Biol Chem 1992, 267:1498-1503 [PubMed] [Google Scholar]
- 62.Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, Perruchoud AP: Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol 1997, 16:398-406 [DOI] [PubMed] [Google Scholar]