Abstract
Fibulin-1, an extracellular matrix protein, is secreted by human ovarian metastatic cancer cell lines under estrogen stimulation. Fibulin-1 expression was quantified by immunohistochemistry and computer-aided image analysis in 44 human ovarian epithelial tumors and 14 normal ovaries. The fibulin-1 staining intensity in proximal stroma, close to the surface of epithelial cells and tumor cells, progressively increased from normal ovaries to serous carcinomas. In all lesions, excluding cystadenomas, fibulin-1 accumulation was higher in proximal stroma than in distant stroma. In situ hybridization demonstrated strong fibulin-1 gene expression in epithelial cells of serous ovarian carcinomas and some cysts. The weak expression of fibulin-1 RNA in some stromal cells of these tumors could not explain the strong fibulin-1 protein accumulation in tumor stroma, which was therefore mostly produced by tumor epithelial cells. In carcinomas, fibulin-1 staining was not correlated with the percentage of estrogen receptor-α (ERα)-stained nuclei but was inversely correlated with the progesterone receptor. However, in cystadenomas and borderline tumors, both fibulin-1 and ERα protein levels increased, in comparison with normal ovaries, suggesting an effect of estrogens in the early steps of tumorigenesis. This fibulin-1 overexpression, demonstrated in vivo in ovarian carcinomas, might be a useful indicator for predicting cancer risk and/or aggressiveness.
Ovarian carcinoma is the fourth most common cause of death due to cancer in women, and its incidence is increasing in developed countries. As ovarian cancers are asymptomatic in their early stages, they have most often spread in the peritoneal cavity at the time of diagnosis. 1 Early detection of premalignant lesions is a particularly important preventive action as ovarian cancers have a very poor prognosis. There is an urgent need for predictive marker(s) that could be assayed in tissues and possibly in plasma or peritoneal exudates. 2 The physiopathology of epithelial ovarian cancers, which originate from the surface epithelium of ovaries, is still poorly understood. Although ∼60% of ovarian carcinomas are estrogen receptor (ER) positive, as measured by classical cytosolic assays, and as invasive ovarian cancers are generally resistant to anti-estrogen therapy, the role of estrogens in ovarian carcinogenesis is controversial. 3-7 This role could be investigated by measuring ER and estrogen-regulated proteins in ovarian tissue at different steps of tumor progression. Studies on ER-positive ovarian cancer cell lines have detected some (but not all) of the estrogen-induced proteins that have been described in breast cancer, eg, cathepsin D, 8 progesterone receptor (PgR), and some transcription factors. 9,10 By contrast, the estrogen-regulated fibulin-1, an extracellular matrix protein, is secreted by ER-positive ovarian cancer cell lines but not by breast cancer cell lines. 8,11 Fibulin-1 is a calcium-binding and acidic glycoprotein of blood and the extracellular matrix. 12 The gene is located at chromosome band 22q13.3. 13 This protein is mostly produced by mesenchymal and endothelial cells in normal tissues. 14 It interacts with fibronectin, 15 fibrinogen, 16 and laminin but not with heparin. Its function is unknown, but it accumulates in the basement membrane with fibronectin and may be involved in cell-matrix interactions.
The aim of this pilot study was to determine the origin in vivo of fibulin-1 (stromal, epithelial, or blood), its significance in tumor progression, and its possible correlation with estrogen and progesterone receptors. We studied the distribution and levels of fibulin-1, ERα, and PgR protein by immunohistochemistry in 14 normal ovaries and 44 human ovarian epithelial tumors. In some tumors, we defined cells producing fibulin-1 mRNA by in situ hybridization.
Materials and Methods
Patients and Ovarian Tissue Sampling
Ovarian tumors were obtained from the Departments of Gynecology and Surgery of the University Hospital of Montpellier, France. After surgery, tumors for immunohistochemical analysis were routinely fixed in formaldehyde (40%; 0.1:1, v/v), acetic acid (100%; 0.1:1, v/v), and methanol (100%; 0.4:1, v/v) in distilled water (FAAM) for at least 24 hours and embedded in paraffin. Thirty-three ovarian epithelial tumors diagnosed between 1994 and 1996 in the Pathology Department included five serous borderline tumors, sixteen serous carcinomas, four mucinous carcinomas, four endometrioid carcinomas, and four clear-cell carcinomas. Eleven serous cystadenomas diagnosed during the same period were selected at random for the same analysis. Fourteen normal ovaries were taken from total hysterectomies performed for prolapsus or fibromyoma. Serous carcinomas and serous cystadenomas to be analyzed by in situ hybridization were immediately frozen in liquid nitrogen after surgical removal and stored at −80°C until in situ hybridization and immunohistochemical analysis of adjacent frozen sections. All tissues were collected for therapeutic or diagnostic purposes according to the ethical rules of Helsinki (1984), modified in Tokyo, and with the approval of the local ethics committee.
Immunohistochemistry
Immunohistochemical analysis was performed on adjacent sections of each paraffin-embedded block of tumors and normal ovaries using control antibody and fibulin-1, ERα, and PgR antibodies, respectively.
Fibulin-1 staining was performed as described with the mouse anti-human monoclonal antibody (MAb) 3A11. 11
ERα was revealed with 1D5 MAb 17 (Dako, Glostrup, Denmark) at 2.5 μg/ml using a microwave antigen retrieval technique. 18 The 1D5 MAb has been directed at the AB domain of the ERα and does not stain ERβ as checked after transfection of ERβ expression vector into MDA-MB231 cells (A. Lucas, unpublished). PgR was revealed with mPRIII (Transbio SARL, Boulogne, France) at 2.5 μg/ml. 19 Staining was performed using a standard streptavidin-biotin enhanced immunoperoxidase technique (LSAB kit, Dako).
Immunostaining specificity was checked using an irrelevant mouse MAb of the same immunoglobulin (Ig) subclass: UPC10 (Sigma) for 3A11 (IgG2a) and MOPC 21 (Sigma) for 1D5 and mPRIII (IgG1). In each experiment, sections of the same FAAM-fixed and paraffin-embedded cell pellet were used as positive external control: BG1 ovarian cancer cell line 20 for Fib-1 staining, ERα-transfected 3Y1-AD12 cell line 21 for ERα staining, and MCF7 for PgR staining.
Fibulin-1 staining was also performed on frozen sections adjacent to sections used for in situ hybridization. The immunostaining technique was the same as that used for the paraffin-embedded samples but with only 1 μg/ml 3A11 MAb and without Pronase treatment.
Immunostaining Quantification
Fibulin-1 staining intensity was quantified with an image analyzer (SAMBA TITN, Unilog, Grenoble, France) adapted to a Leitz DMRB light microscope (Leica, Wetzlar, Germany) and a 3-CCD DXC-950P color video camera (Sony Corp., Tokyo, Japan) connected to a microcomputer. Fibulin-1 staining intensity was quantified in each section (ovarian and/or peritoneal localization) of the 32 serous tumors, both in proximal tumor stroma (stroma located 0 to 550 μm from tumor cells) and in distant stroma (stroma located 550 μm to 1.5 mm from these cells). In normal ovaries, fibulin-1 staining was also quantified in proximal stroma (0 to 550 μm from surface epithelial cells) and in distant stroma. Staining intensity was assessed as the mean integrated optical density measured in each section on 4 to 65 fields (one field corresponding to 550 μm diameter at ×20 magnification). The field number varied with the tumor area and labeling heterogeneity. The background intensity of the negative control in an adjacent section was subtracted from the total intensity to obtain specific staining. 22
No significant variations were observed between experiments, as checked with a positive stained external control (BG1 cells pellet) analyzed in parallel.
ERα and PgR immunostaining semiquantification was performed using light microscopy. The percentage of nuclei from tumor and normal epithelial cells stained with the two antibodies was estimated and separated into three groups with low, moderate, and high receptor content (see Figure 7 ▶ ).
Figure 7.
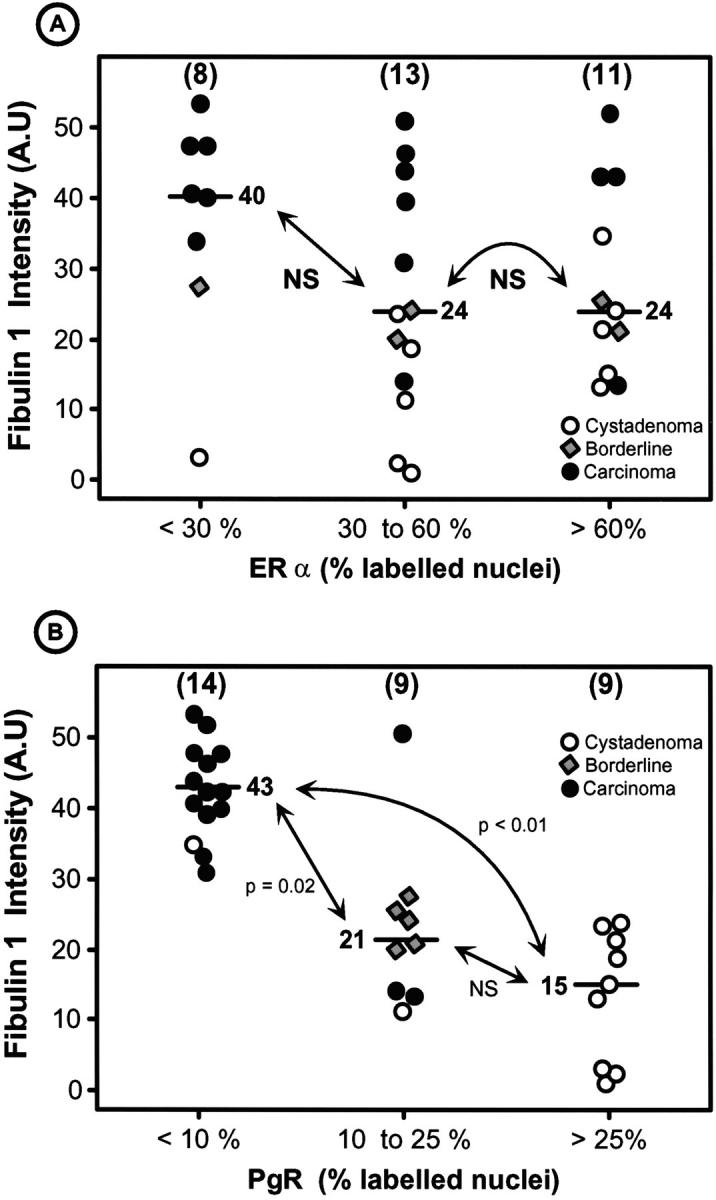
Correlation between fibulin-1 staining intensity in tumor stroma and ERα (A) or PgR (B) nuclear staining in serous tumors. There was only one ERα-negative ovarian carcinoma, whereas 10 of 16 carcinomas were negative for PgR staining. P values were determined according to the nonparametric Kruskas-Wallis test (A) and the nonparametric Wilcoxon-Mann-Whitney test (B). Bar, median value. Fibulin-1 was not correlated with ERα but was negatively correlated with PgR.
In Situ Hybridization
The cellular distribution of fibulin-1 mRNA was analyzed with a nonisotopic in situ hybridization procedure using digoxigenin-labeled probe. A 325-mer antisense probe was chosen in the common region of fibulin-1 from nucleotides 84 to 409. Probe specificity was checked using the GeneJockey program on a Macintosh IICi computer and relative to Gene Bank nucleotide sequences.
The fibulin-1 cDNA template was synthesized by reverse transcription polymerase chain reaction (RT-PCR). One microgram of total RNA from the BG1 cell line was reverse transcribed as described. 23 The PCR primers used were the 5′ end primer CCGGAGTGGACGCGGATG (FibD, 84 to 102 bp) and the 3′ end primer GCCAACCATGAGGCTGTACTCG (FibR, 387 to 409 bp). One microliter of the RT product was amplified by PCR in a final volume of 25 μl containing 20 mmol/L Tris/HCl (pH 8.55), 16 mmol/L (NH4)2SO4, 2.5 mmol/L MgCl2, 50 μmol/L dNTP, with 5 pmol of oligonucleotides, and 0.125 U of Taq DNA polymerase (Bioprobe, Montreuil, France). After an initial 2-minute denaturation step at 94°C, 36 rounds of PCR amplification were carried out on a DNA thermocycler (Perkin Elmer, Courtaboeuf, France). One cycle included 30 seconds denaturation at 94°C, 30 seconds annealing at 60°C, and 1 minute extension at 72°C. The samples underwent a final 7-minute extension step at 72°C. RT-PCR products were eluted from a 0.8% agarose gel using QIAquick gel extraction (QIAgen, Courtaboeuf, France) according to the manufacturer’s instructions. The PCR product was then subcloned in the pGEMT plasmid (Promega, Charbonnières, France).
Digoxigenin-labeled antisense and sense RNA probes were synthesized with the Dig labeling transcription kit according to the manufacturer’s instructions using T7 and SP6 RNA polymerase (Boerhringer Mannheim, Mannheim, Germany). Labeling efficiency was estimated using serial dilutions of the labeled probes that were spotted and fixed on a nylon membrane. The sense probe dilution was adjusted to the antisense probe dilution.
Adjacent 5- to 7-μm tissue sections were collected on Silane-prep slides (Sigma Diagnostics, St. Louis, MO) and fixed with paraformaldehyde (4%) in 0.1 mol/L phosphate buffer (PB; pH 7.4) at room temperature for 30 minutes. Slides were dehydrated in ethanol. In situ hybridization was performed as described. 24 The antisense and sense probes were added to the tissue at a final concentration of 40 ng/μl in 20 μl of hybridization buffer. Hybridization was carried out at 42°C overnight in a humid chamber. Slides were incubated with polyclonal sheep anti-digoxigenin Fab fragments conjugated with calf intestinal alkaline phosphatase (Boehringer Mannheim), diluted 1:1000 at room temperature for 120 minutes. Color slides were developed overnight using nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indoyphosphate in the presence of 24 mg/100 ml of levamisole. Slides were mounted with Glycergel (Dako, Carpinteria, CA) and stored in a dark box at 4°C.
For each sample, slides without any probe, sections hybridized with sense oligonucleotide probe, and slides treated with RNAse A before hybridization were used as nonspecific staining controls. mRNA integrity was controlled by amplification of the GAPDH housekeeping gene on adjacent sections.
Statistical Analysis
The data were analyzed using the STAT-ITCF package (Institut Technique de Céréales et des Fourrages, Paris, France).
Results
Fibulin-1 Staining
In normal ovaries, fibulin-1 was generally weakly stained in the stroma close to surface epithelial cells and strongly stained in the vascular vessel walls.
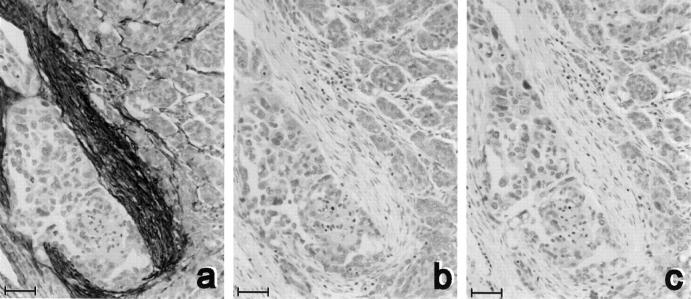
In most ovarian tumors, fibulin-1 was found in the stroma and not in tumor epithelial cells (Figure 1a) ▶ . The specificity of this stromal staining was shown using irrelevant antibody 11 (Figure 1c) ▶ and extinction by the addition of pure fibulin-1 in excess (Figure 1b) ▶ .
Figure 1.
Specificity of immunohistochemical staining of fibulin-1. Adjacent sections from a serous ovarian carcinoma were treated with 2 μg/ml of the 3A11 anti-human fibulin-1 MAb alone (a), with a 100-fold excess of pure fibulin-1 (b), or with an irrelevant antibody (2 μg/ml UPC 10; (c)). Counterstaining was with hematoxylin. Bar, 50 μm.
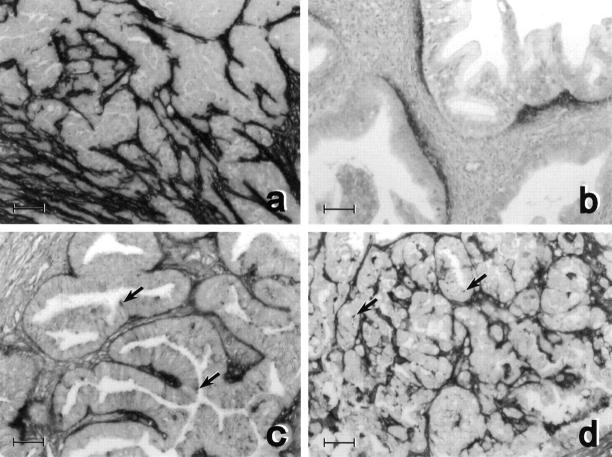
In all ovarian carcinomas, fibulin-1 was mostly accumulated in stroma close to cancer cells. Staining was generally very strong in serous carcinomas and slighter in mucinous and endometrioid carcinomas (Figure 2) ▶ .
Figure 2.
Fibulin-1 staining in four types of ovarian carcinomas: serous (a), mucinous (b), endometrioid (c), and clear-cell (d). In all tumors, the stroma close to cancer cells was labeled. Staining was not detected in cancer cells of serous (a) and mucinous (b) carcinomas. Punctuate staining was noted in some cancer cells (arrows) of clear-cell (d) and endometrioid (c) carcinomas, where apical staining was also detected. Counterstaining was with hematoxylin. Bar, 50 μm.
Staining was generally not detected in cancer cells, except in 1 of the 16 serous carcinomas where weak staining was also noted in some of the cancer cells (data not shown). Staining was punctuated in some cancer cells of clear-cell carcinomas and mostly apical in four endometrioid carcinomas. In mucinous carcinomas, a fibulin-1 gradient was clearly noted in stroma with a maximal intensity close to cancer cells (Figure 2b) ▶ . In addition, vascular vessels were strongly stained with fibulin-1 in all tumors and in normal ovaries, as previously reported for most tissues. 14
Fibulin-1 Staining Quantification
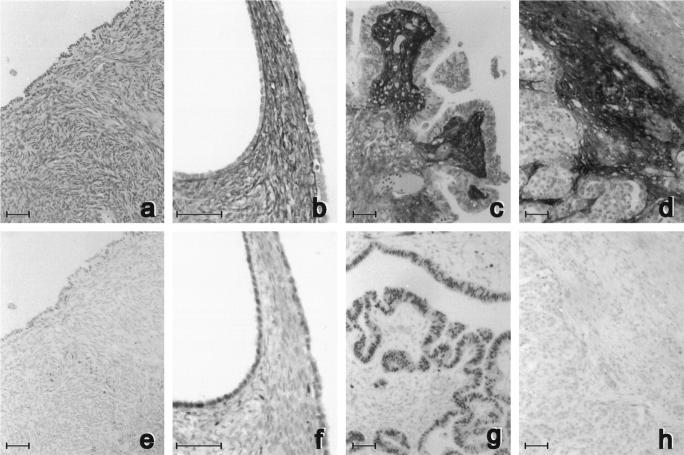
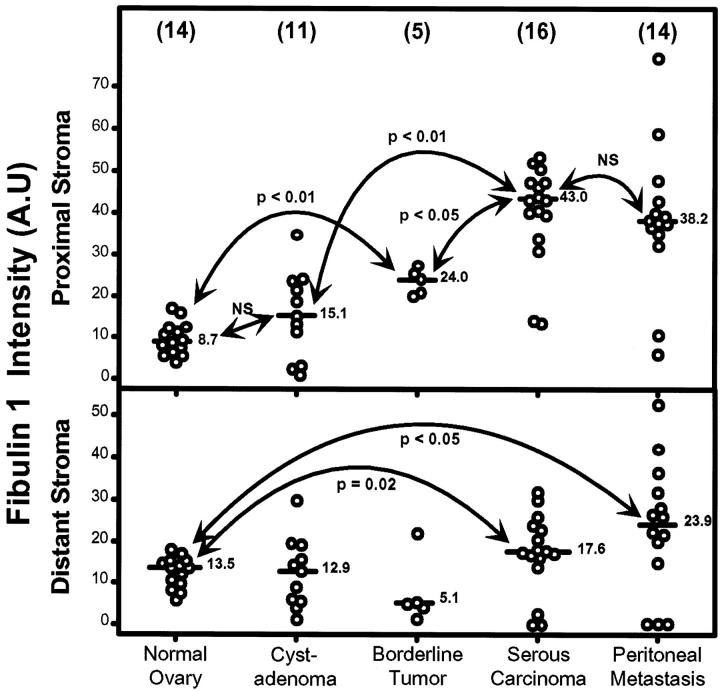
As serous ovarian carcinomas are the most common epithelial ovarian tumors (46%), 25 we quantified fibulin-1 staining only in serous-type tumors and compared it with staining in normal ovary. Even though the staining intensity varied for different patients, Figure 3 ▶ is representative of the general trend for these four types of ovarian tissues. Fibulin-1 staining was nil or weak in stroma of normal ovaries. It increased in some cystadenomas, with stronger staining intensity close to the basement membrane of surface epithelial cells. Staining was markedly higher in the stroma of papillary structures of all borderline tumors, ie, stronger under the proliferative surface epithelial cells than in distant stroma. In serous carcinomas, it was also stronger in peritumor stroma than in distant stroma. To assess the significance of this apparent increase in fibulin-1 accumulation during tumor progression in these four types of tissue, we systematically quantified fibulin-1 intensity in stroma close to surface epithelial cells and serous carcinoma cells and compared it with intensities in distant stroma, as described in Materials and Methods. As shown in Figures 4 and 5 ▶ ▶ , mean fibulin-1 staining was stable in normal ovaries and in most serous cystadenomas and not significantly higher in proximal stroma than in distant stroma. In normal ovaries, the slightly higher intensity in distant stroma suggests that fibulin-1 normally originates more from blood than from surface epithelial cells.
Figure 3.
Adjacent sections of normal ovaries (a and e) serous cystadenomas (b and f), borderline tumors (c and g), and carcinomas (d and h) stained with anti-fibulin-1 3A11 antibody (a to d) or with the anti-estrogen receptor-α 1D5 antibody (e to h). Counterstaining was with hematoxylin. Bar, 50 μm.
Figure 4.
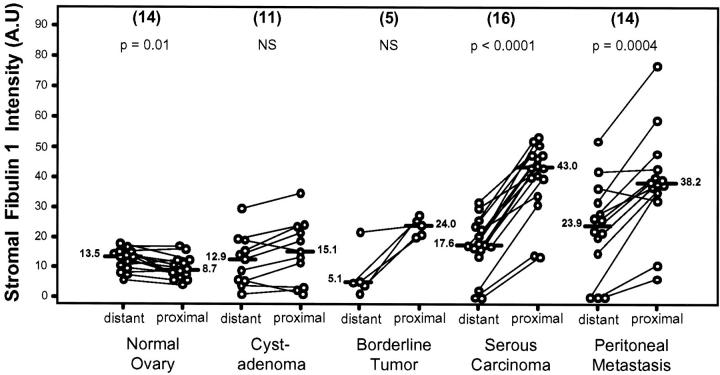
Comparison of fibulin-1 staining intensity expressed in arbitrary units (A.U.) in proximal and distant stroma of normal ovaries, serous cystadenomas, borderline tumors, serous carcinomas, and metastases. The number of different samples is in brackets. Bar, median value. For serous carcinomas, the mean value was used when two localizations were quantified. P values were determined according to the nonparametric Wilcoxon test for paired values.
Figure 5.
Fibulin-1 staining intensity quantification in arbitrary units (A.U.) in proximal stroma (upper) and distant stroma (lower) of normal ovaries, serous cystadenomas, borderline tumors, carcinomas, and corresponding peritoneal metastases. P values were determined according to the nonparametric Wilcoxon-Mann-Whitney test. Bar, median value. Number of different samples is shown in parentheses.
By contrast, in almost all borderline tumors, serous carcinomas and metastases, proximal stromal staining was higher than distant staining (Figure 4) ▶ , strongly suggesting that fibulin-1 characterizes a stroma built by tumor cells. A comparison of median values for these five types of lesions (Figure 5) ▶ revealed a significant increased fibulin-1 staining in tumor stroma, starting in borderline tumors and reaching maximal intensity in serous carcinomas and peritoneal metastases. This fibronectin-binding protein was not or only slightly detected in epithelial cells but mostly accumulated in the extracellular matrix close to tumor cells. These results suggested that fibulin-1 overexpression during ovarian tumorigenesis originated from surface epithelial cells and/or stromal cells surrounding the tumor. Fibulin-1 staining intensity in distant stroma was stable in premalignant lesions but significantly increased in serous carcinomas and peritoneal metastases (Figure 5) ▶ . It is unknown whether fibulin-1 accumulation in distant stroma originates from tumor cells or ascites.
Fibulin-1 staining intensity was similar in the different serous carcinoma localizations (right and/or left ovary, peritoneal metastases) in the same patient but ranged from 10 to 70 arbitrary units in different patients (not shown). This suggested that increased fibulin-1 accumulation in stroma is a characteristic of epithelial tumor cells and indicated that this rapidly invasive cancer has the same clonal origin and characteristics regardless of whether it is located in the primary tumor or a peritoneal metastasis.
In Situ Hybridization of Fibulin-1 RNA
To determine the cell type expressing fibulin-1 gene, we then performed in situ hybridization on frozen sections of some serous ovarian tumors (cystadenomas and carcinomas). Hybridization specificity was controlled in sections hybridized with sense oligonucleotide probe (Figure 6, a, d, and g) ▶ , sections without probe, and sections treated with RNAAse A before hybridization (not shown).
Figure 6.
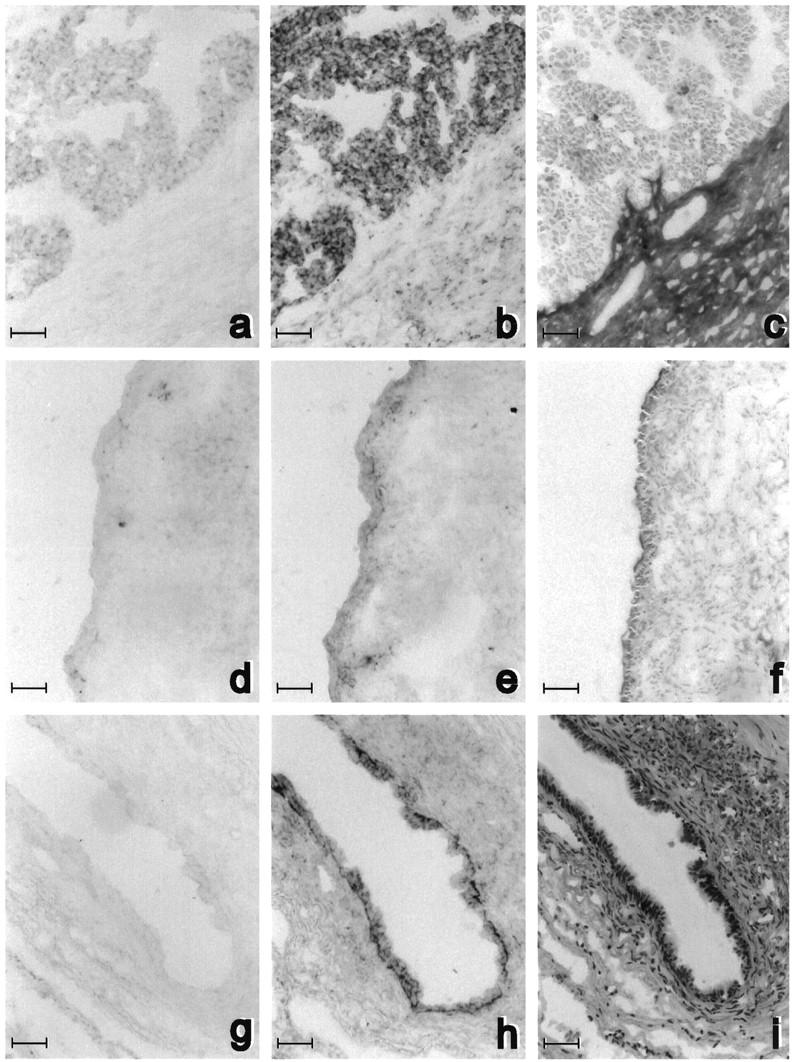
Fibulin-1 RNA expression in adjacent frozen sections of one serous ovarian carcinoma (a to c) and two serous cystadenomas (d to i). In situ hybridization was performed without counterstaining with fibulin-1 antisense probe (b, e, and h) and with sense probe (a, d, and g) as negative control. Adjacent sections were stained with anti-fibulin-1 3A11 antibody (c and f) and with hematoxylin and eosin (i). Bar, 50 μm.
In carcinomas (Figure 6b) ▶ , strong fibulin-1 mRNA expression was detected in most epithelial cancer cells. Fibulin-1 immunostaining on adjacent sections was strong in stroma close to cancer cells (Figure 6c) ▶ and higher than in distant stroma, as described above in other tumors. In one cyst, fibulin-1 expression was low in epithelial and stromal cells (Figure 6f) ▶ . Conversely, in another cyst, there was strong fibulin-1 mRNA expression in the epithelial cell layer within the cyst lumen. The stroma bordering this cyst contained many cells, as shown by hematoxylin and eosin staining (Figure 6i) ▶ , and was strongly stained for fibulin-1 protein (not shown). However, only some stromal cells expressed fibulin-1 mRNA (Figure 6h) ▶ , indicating that fibulin-1 in this case was mostly produced by epithelial cells. Altogether, these results demonstrated that fibulin-1 mRNA was mainly produced by tumor epithelial cells but that accumulation of the corresponding protein in proximal stroma was correlated with fibulin-1 mRNA expression in the tumor.
Correlation between Fibulin-1 Stromal Staining and Other Prognostic Clinico-Pathological Variables
Fibulin-1 staining intensity in tumor stroma was not correlated with any other prognostic variables such as lymph node involvement, histological grade, FIGO stage, or age of patients. However, most ovarian carcinomas were graded as FIGO stage 3 or 4, whereas only two carcinomas were stage 1 and 2, respectively. The clinical follow-up time of 1 to 40 months was not sufficient to evaluate the putative prognostic value of fibulin-1 staining. As fibulin-1 is known to be induced in vitro by estradiol in ovarian cancer cell lines, we also studied ERα and PgR staining in serial sections of the same tumors.
Estrogen Receptor-α
Figure 3 ▶ (e, f, g, and h) shows one representative example of ERα staining in sections adjacent to fibulin-1 staining in normal ovary and ovarian tumors. ERα was generally weakly stained in the nucleus of surface epithelial cells in normal ovaries. In cystadenomas and borderline tumors, ERα staining intensity and the percentage of stained nuclei were generally higher, as summarized in Table 1 ▶ for all samples. ERα staining was generally weak in normal ovaries, even though normal surface epithelial cells were often detached and lost.
Table 1.
Estrogen Receptor-α and Progesterone Receptor Staining in Ovary and Serous Ovarian Tumors
| % labeled nuclei | Normal ovary (10) | Cystadenoma (11) | Borderline tumors (5) | Carcinoma (16) |
|---|---|---|---|---|
| ERα 0 to 29% | 10 (100%) | 1 (9%) | 1 (20%) | 6 (37.5%) |
| 30 to 60% | 0 (0%) | 5 (45.5%) | 2 (40%) | 6 (37.5%) |
| 61 to 100% | 0 (0%) | 5 (45.5%) | 2 (40%) | 4 (25%) |
| PgR 0 to 9% | 6 (60%) | 1 (9%) | 0 (0%) | 13 (81%) |
| 10 to 25% | 0 (0%) | 1 (9%) | 5 (100%) | 3 (19%) |
| 26 to 100% | 4 (40%) | 9 (82%) | 0 (0%) | 0 (0%) |
Legend: The percentage of positive nuclei for ERα and PgR staining was determined as described in Materials and Methods and Figure 7 ▶ . Only 10 of the 16 normal ovaries analyzed for fibulin-1 were analyzed for ER and PgR, as surface epithelial cells were detached by fixation in 6 of them.
Figure 7A ▶ shows the absence of correlation between fibulin-1 stromal staining intensity and nuclear ERα staining in serous cystadenomas, borderline tumors, and carcinomas. This absence of correlation was clearest in carcinomas that most often overexpressed fibulin-1 but weakly expressed ERα in 37% of cases. When normal ovaries, cysts, and borderline tumors were pooled, there was a trend for a positive correlation between these two markers, as the fibulin-1 staining intensity was significantly lower in the low ERα group than in the high ERα group (P < 0.01). This suggests that fibulin-1 might be estrogen dependent in the early steps of ovarian tumorigenesis, as discussed later.
Progesterone Receptor Staining
As shown in Table 1 ▶ , and Figure 7B ▶ , PgR-positive cells were significantly lower in serous carcinomas in comparison with borderline and serous cysts. A significant inverse correlation was therefore obtained with fibulin-1 that, conversely, increased with tumor progression (Figure 7B) ▶ . Carcinomas were mostly low in PgR and high in fibulin-1, with 10 of 16 carcinomas being negative for PgR staining. Cystadenomas had high PgR and low fibulin-1 levels, and borderline tumors had intermediate PgR content. PgR staining was variable in the 10 normal ovaries represented in Table 1 ▶ .
Discussion
We evaluated fibulin-1 levels in 14 normal human ovaries and 44 ovarian epithelial tumors and determined the percentage of ERα- and PgR-positive cells in adjacent sections.
The first information highlighted by our study concerns the site of fibulin-1 synthesis and accumulation in ovarian tumors. As expected on the basis of previous studies, fibulin-1 was mostly accumulated in stroma due to its high affinity to components of the extracellular matrix, and also stained vessel walls. As fibulin-1 circulates in blood 12 and is secreted by ovarian epithelial cancer cell lines, 8,11 we investigated whether its accumulation in stroma originated from blood, epithelial tumor cells, or stromal cells. Our finding that, in ovarian tumors, fibulin-1 staining intensity was significantly higher in stroma close to tumor cells than in distant stroma close to large vessels strongly suggested a tumor cell origin. This was demonstrated by in situ hybridization, which showed strong fibulin-1 transcript expression in the epithelial cell layer of some cystadenoma lumens and in serous ovarian cancer cells. Some stromal cells (likely fibroblasts) also expressed fibulin-1 RNA.
The fact that cellular epithelial staining of the protein was rarely obtained is in agreement with in vitro results on BG-1 cells that overexpress fibulin-1 RNA (data not shown) and secrete the corresponding protein. 11 Immunostaining of fibulin-1 in these cells was positive on the fibronectin-coated substrate but weak in BG-1 cells, indicating that fibulin-1 is mainly secreted (our unpublished results). However, the additional contribution of fibroblasts in stroma close to tumors, which would be selectively stimulated by paracrine factors from cancer cells to secrete fibulin-1, is not excluded. In some cystadenomas and some borderline tumors, intense and linear fibulin-1 staining under the basal pole of surface epithelial cells indicated that fibulin-1 is a basement membrane component formed by epithelial tumor cells.
The second important finding is that fibulin-1 stromal staining close to tumor cells or surface epithelial cells progressively increased during ovarian tumor progression, starting in borderline tumors and peaking in serous ovarian cancers. The progressive accumulation of fibulin-1 during the stromal reaction of the tumor is most likely due to increased fibulin-1 gene expression, as observed here in tumor cells by in situ hybridization and as described previously in cancer cell lines. 11 Further increased expression of a fibulin-1 binding protein such as fibronectin, as observed in some ovarian cancers, 26 was considered. However, immunohistochemical staining of fibronectin in adjacent sections of two serous ovarian cancers showed that fibronectin and fibulin-1 were not always associated and that fibronectin was not specifically accumulated in proximal stroma, contrary to fibulin-1 (our unpublished data). The increased fibulin-1 expression and accumulation observed at the RNA and protein level in some serous cystadenomas might have prognostic significance in terms of risk markers in these cysts. This will require additional studies and clinical follow-up.
The possible consequence in tumor invasion of the increased fibulin-1 accumulation in the stroma of ovarian tumors is an interesting hypothesis. The fact that this secreted protein interacts strongly with components of the extracellular matrix suggests that it could modulate their effect on cell attachment and/or cell motility and could participate in tissue remodeling. Tenascin, another fibronectin-binding protein, was also shown to be accumulated close to breast cancer cells 27,28 and associated with poor prognosis in breast cancer. 28 Conversely, recent results from our laboratory showed an inhibitory effect of fibulin-1 on cancer cell motility stimulated by fibronectin in a two-chamber multi-well system, 29 and others have suggested that fibulin-1 could be coded by a tumor suppressor gene. 30 Investigations on mutations, loss of heterozygosity, or alternative splicing of fibulin-1 as well as clinical follow-up on a larger number of patients are required to determine whether or not local fibulin-1 overexpression interferes positively or negatively, or is only associated with ovarian tumor aggressiveness.
A third finding concerns the absence of correlation between the estrogen-regulated fibulin-1 and ERα level in ovarian tumors. This lack of correlation was mostly observed with serous carcinomas and suggests that at this late step of carcinogenesis, fibulin-1 may be regulated by signals other than estrogens, eg, growth factors, as also observed for estrogen-regulated cathepsin D in breast cancer. 31 The major involvement of ERβ, which is known to be more expressed in ovaries than ERα, 32 but which is not detected by the ER antibody used in this study, should also be considered. ERα was mostly expressed in surface epithelial cells of ovaries and also in some stromal cells. It was present in 43% of all ovarian carcinomas, in agreement with previous studies on ERα immunohistochemical staining 3,33 and the results of cytosolic assays. 3,7 However, at earlier steps of tumorigenesis, in cystadenomas and borderline tumors, there was concomitant increased expression of both ERα and fibulin-1 relative to normal ovaries. Moreover, the trend indicated a positive correlation between fibulin-1 and ERα in cystadenomas, and this was more significant when the results for normal ovaries, cysts, and borderline tumors were pooled. On the basis of the estrogen-enhanced fibulin-1 expression demonstrated in three ER-positive ovarian cancer cell lines 11 and of the mitogenic activity of estradiol in these cells and in normal ovarian surface epithelial cells, 34 it is tempting to speculate that estrogens have the same mitogenic effect in vivo during the early steps of ovarian tumorigenesis. Increased local estrogenic activity, facilitated by an increased ERα content, as shown here and previously 35 in some cystadenomas and borderline tumors, may also be responsible for fibulin-1 overexpression and secretion, thus participating in tissue remodeling by preparing the tumor stroma. However, fibulin-1 was also overexpressed in ER-negative serous carcinomas, explaining the lack of correlation between these two markers at later stages of carcinogenesis. The significant inverse correlation between two estrogen-regulated proteins, fibulin-1 and PgR, in ER-positive cells suggests that these two genes are differently regulated by the estrogen receptor (different cross-talk, different ERα or -β species, and/or different coactivators involved in these regulations?).
To conclude, this study clearly shows that fibulin-1 stromal staining in ovarian serous tumors originates at least partly from surface epithelial cells and cancer cells that produce the corresponding mRNA. Fibulin-1 overexpression and secretion at the basal pole of these cells may be associated with ovarian tumorigenesis but cannot be considered as a marker of estrogen responsiveness in ovarian cancers. Additional clinical studies and a complete understanding of fibulin-1 function in the extracellular matrix will be required to determine its significance as a prognostic marker. This study should prompt additional biological and clinical work on a larger number of patients including clinical follow-up in an attempt to determine the value of fibulin-1 as a marker of premalignant lesions and/or aggressiveness.
Acknowledgments
We are grateful to Dr. Scott Argraves and Dr. Svetlana Godyna (Medical University of South Carolina, Charleston, SC) for providing the 3AII antifibulin-1 antibody and pure human fibulin-1. We thank Prof. F. Laffargue for providing the ovarian tumors, Profs. T. Maudelonde and J. P. Daures for statistical analysis and are grateful to Jean-Yves Cance for the photographic artwork, along with Fabienne David and Nadia Kerdjadj for secretarial assistance.
Footnotes
Address reprint requests to Dr. Henri Rochefort, INSERM U.148, Department of Cell Biology, 60, rue de Navacelles, 34090 Montpellier, France. E-mail: rochefor@u148.montp.inserm.fr.
Supported by the Institut National de la Santé et de la Recherche Médicale, the Faculty of Medicine and University Hospital of Montpellier, the Association pour la Recherche sur le Cancer, the Groupement des Entreprises Française dans la Lutte Contre le Cancer, and the Ligue Nationale Contre le Cancer.
References
- 1.Young RC, Knapp RC, Perez CA: Cancer of the ovary. De Vita VT Hellman S Rosenberg SA eds. Principles and Practice of Oncology. 1985, :pp 884-913 Lippincot, Philadelphia [Google Scholar]
- 2.Granai CO: Ovarian cancer: unrealistic expectation. N Engl J Med 1992, 327:197-199 [DOI] [PubMed] [Google Scholar]
- 3.Kommoss F, Pfisterer J, Thome M, Schäfer W, Sauerbrei W, Pfleiderer A: Steroid receptors in ovarian carcinoma: immunohistochemical determination may lead to new aspects. Gynecol Oncol 1992, 47:317-322 [DOI] [PubMed] [Google Scholar]
- 4.Rao BR, Slotman BJ: Endocrine factors in common epithelial ovarian cancer. Endocr Rev 1991, 12:14-26 [DOI] [PubMed] [Google Scholar]
- 5.Willcocks D, Toppila M, Hudson CN, Tyler JP, Baird PJ, Eastman CJ: Estrogen and progesterone receptors in human ovarian tumors. Gynecol Oncol 1983, 16:246-253 [DOI] [PubMed] [Google Scholar]
- 6.Harding M, Cowan S, Hole D, Cassidy L, Kitchener H, Davis J, Leake R: Estrogen and progesterone receptors in ovarian cancer. Cancer 1990, 65:486-491 [DOI] [PubMed] [Google Scholar]
- 7.Isola J, Kallionemi OP, Korte JM, Wahlström T, Aine R, Helle M, Helin H: Steroid receptors and Ki-67 reactivity in ovarian cancer and in normal ovary: correlation with DNA flow cytometry, biochemical receptor assay, and patient survival. J Pathol 1990, 162:295-301 [DOI] [PubMed] [Google Scholar]
- 8.Galtier-Dereure F, Capony F, Maudelonde T, Rochefort H: Estradiol stimulates cell growth and secretion of pro-cathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J Clin Endocrinol Metab 1992, 75:1497-1502 [DOI] [PubMed] [Google Scholar]
- 9.Langdon SP, Hirst GL, Miller EP, Hawkins RA, Tesdale AL, Smyth JF, Miller WR: The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol 1994, 50:131-135 [DOI] [PubMed] [Google Scholar]
- 10.Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM: SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J Steroid Biochem Mol Biol 1995, 55:279-289 [DOI] [PubMed] [Google Scholar]
- 11.Clinton GM, Rougeot C, Derancourt J, Roger P, Defrenne A, Godyna S, Argraves WS, Rochefort H: Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. Proc Natl Acad Sci USA 1996, 93:316-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argraves WS, Tran H, Burgess WH, Dickerson K: Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J Cell Biol 1990, 111:3155-3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korenberg JR, Chen XN, Tran H, Argraves WS: Localization of the human gene for fibulin-1 (FBLN1) to chromosome band 22q13.3. Cytogenet Cell Genet 1995, 68:192-193 [DOI] [PubMed] [Google Scholar]
- 14.Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS: The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J Histochem Cytochem 1995, 43:401-411 [DOI] [PubMed] [Google Scholar]
- 15.Godyna S, Mann DM, Argraves WS: A quantitative analysis of the incorporation of fibulin-1 into extracellular matrix indicates that fibronectin assembly is required. Matrix Biol 1995, 14:467-477 [DOI] [PubMed] [Google Scholar]
- 16.Tran H, Tanaka A, Litvinovich SV, Medved LV, Haudenschild CC, Argraves WS: The interaction of fibulin-1 with fibrinogen: a potential role in hemostasis and thrombosis. J Biol Chem 1995, 270:19458-19464 [DOI] [PubMed] [Google Scholar]
- 17.Al Saati T, Clamens S, Cohen-Knafo E, Faye JC, Prats H, Coindre JM, Wafflart J, Caverivière P, Bayard F, Delsol G: Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer 1993, 55:651-654 [DOI] [PubMed] [Google Scholar]
- 18.Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, Bell J, Elston CWE, Robertson JF, Blamey RW, Ellis IO: A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol 1995, 26:291-294 [DOI] [PubMed] [Google Scholar]
- 19.Vu Hai MT, Jolivet A, Ravet V, Lorenzo F, Perrot-Applanat M, Citerne M, Milgrom E: Novel monoclonal antibodies against human uterine progesterone receptor. Biochem J 1989, 260:371-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisinger KR, Kute TE, Pettenati MJ, Welander CE, Dennard Y, Collins LA, Berens ME: Characterization of a human ovarian carcinoma cell line with estrogen and progesterone receptors. Cancer 1989, 63:280-288 [DOI] [PubMed] [Google Scholar]
- 21.Garcia M, Derocq D, Platet N, Bonnet S, Brouillet JP, Touitou I, Rochefort H: Both estradiol and tamoxifen decrease proliferation and invasiveness of cancer cells transfected with a mutated estrogen receptor. J Steroid Biochem Mol Biol 1997, 61:11-17 [DOI] [PubMed] [Google Scholar]
- 22.Maudelonde T, Brouillet JP, Roger P, Giraudier V, Pages A, Rochefort H: Immunostaining of cathepsin D in breast cancer: quantification by computerised image analysis and correlation with cytosolic assay. Eur J Cancer 1992, 28A:1686-1691 [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T: Reverse transcriptase (RNA-dependent DNA polymerase). Sambrook J eds. Molecular Cloning: A Laboratory Manual, 1989, vol 1.:pp 5.52-5.57 Cold Spring Harbor Laboratory Press Cold Spring Harbor, NY [Google Scholar]
- 24.Spector DL, Goldman RD, Leinwand LA: In situ hybridization to RNA cells: subcellular localization of genes and their products. Spector DL eds. Cells: A Laboratory Manual, 1998, vol 3.:pp 116.1-116.16 Cold Spring Harbor Laboratory Press Cold Spring Harbor, NY [Google Scholar]
- 25.Krigman H, Bentley R, Robboy SJ: Pathology of epithelial ovarian tumors. Clin Obstet Gynaecol 1994, 37:475-491 [DOI] [PubMed] [Google Scholar]
- 26.Menzin AW, Loret de Mola JR, Bilker WB, Wheeler JE, Rubin SC, Feinberg RF: Identification of oncofetal fibronectin in patient with advanced epithelial ovarian cancer: detection in ascitic fluid and localization to primary sites and metastatic implants. Cancer 1998, 82:152-158 [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Matsumoto EI, Hanamura N, Kalembeyi I, Katsuta K, Ishihara A, Sakakura T: Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 1997, 182:421-428 [DOI] [PubMed] [Google Scholar]
- 28.Jahkola T, Toivonen T, Von Smitten K, Blomqvist C, Virtanen I: Expression of tenascin in invasion border of early breast cancer correlates with higher risk of distant metastasis. Int J Cancer 1996, 69:445-447 [DOI] [PubMed] [Google Scholar]
- 29.Hayashido Y, Lucas A, Rougeot C, Godyna S, Argraves WS, Rochefort H: Estradiol and fibulin-1 inhibit motility of human ovarian and breast cancer cells induced by fibronectin. Int J Cancer 1998, 75:654-658 [DOI] [PubMed] [Google Scholar]
- 30.Qing J, Maher VM, Tran H, Argraves WS, Dunstan RW, McCormick JJ: Suppression of anchorage-independent growth and Matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene 1997, 15:2159-2168 [DOI] [PubMed] [Google Scholar]
- 31.Cavailles V, Garcia M, Rochefort H: Regulation of cathepsin D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol Endocrinol 1989, 3:552-558 [DOI] [PubMed] [Google Scholar]
- 32.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA: Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997, 138:863-870 [DOI] [PubMed] [Google Scholar]
- 33.Henzen-Logmans SC, Fieret EJ, Berns EM, Van der Burg ME, Klijn JG, Foekens JA: Ki-67 staining in benign, borderline, malignant primary and metastatic ovarian tumors: correlation with steroid receptors, epidermal-growth-factor receptor and cathepsin D. Int J Cancer 1994, 57:468-472 [DOI] [PubMed] [Google Scholar]
- 34.Gondos B: Surface epithelium of developing ovary. Am J Pathol 1975, 81:303-320 [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Jawdeh GM, Jacobs TW, Niloff J, Cannistra SA: Estrogen receptor expression is a common feature of ovarian borderline tumors. Gynecol Oncol 1996, 60:301-307 [DOI] [PubMed] [Google Scholar]