Abstract
The E-cadherin-catenin complex, by mediating intercellular adhesion, regulates the architectural integrity of epithelia. Down-regulation of its expression is thought to contribute to invasion of carcinoma cells. To investigate the involvement of the E-cadherin-catenin adhesion system in the progression of human bronchopulmonary carcinomas, we compared the immunohistochemical distribution of E-cadherin, α-catenin, and β-catenin in four human bronchial cancer cell lines with different invasive abilities and in 44 primary bronchopulmonary tumors. Although invasive bronchial cell lines did not express E-cadherin and α-catenin, complete down-regulation of cadherin-catenin complex expression was a rare event in vivo in bronchopulmonary carcinomas. Nevertheless, a spotty and cytoplasmic pattern of E-cadherin and catenins was observed in 32 primary tumors, only in invasive tumor clusters. Immunoprecipitation experiments showed that this redistribution was not related to a disruption of cadherin-catenin interaction but to down-regulated tyrosine phosphorylation of E-cadherin. We conclude that loss of E-cadherin and/or catenins is not a prominent early event in the invasive progression of human bronchopulmonary carcinomas in vivo. The decreased tyrosine phosphorylation of E-cadherin may reflect a loss of functionality of the complex and implicates a major role in tumor invasion.
Tissue homeostasis and morphogenesis are regulated by cell adhesion molecules expressed on the cell surface and comprising four large groups: the integrin family, cadherins, the immunoglobulin superfamily, and selectins. 1,2 Among these molecules, transmembrane glycoprotein cadherins play a major role in intercellular connection. 2-4 E-cadherin is a member of the cadherin family that mediates homotypic calcium-dependent cell-cell adhesion to ensure the maintenance of a normal phenotype of epithelial cells. 5,6 The specific cytoplasmic domain of E-cadherin interacts with catenin molecules, which establish an intracellular linkage with the actin cytoskeleton. Catenins comprise at least three molecules, α-, β-, and γ-catenin, and are able to form at least two different trimeric adhesion complexes that include both E-cadherin and α-catenin and either β-catenin or γ-catenin (plakoglobin) in a mutually exclusive manner. 6-8 The full adhesive function of E-cadherin depends on the integrity of the entire cadherin-catenin-actin network. Indeed, decrease of adhesive properties of cadherin-catenin complex has been shown to be related to the loss of differentiation and the subsequent acquisition of a higher motility and invasiveness of epithelial cells. 9 The dysfunction in E-cadherin-mediated adhesion can be generated by several mechanisms, including decrease or lack of E-cadherin or catenin expression, mutations and deletions in the genes encoding E-cadherin or catenins, and posttranslational alterations such as aberrant tyrosine phosphorylation of the E-cadherin-catenin complex. 6,7
Consequently, it has been suggested that the cadherin-catenin complex function plays a critical role in the pathogenesis of human carcinomas. 2,7,9 Several in vitro studies have demonstrated an invasion-suppressor role for E-cadherin and catenins by showing a strong correlation between the defect of cadherin-catenin complex expression and both loss of the epithelial phenotype and increase of the invasive phenotype. 10-13 Moreover, restoration of E-cadherin or catenins levels by cDNA transfection experiments leads to the recovery of the epithelial phenotype, decrease of invasiveness, and tumorigenic and metastatic capability of cultured tumor cells. 14-18 In vivo results are not so clear-cut. Indeed, the bulk of morphological studies have suggested an inverse correlation between E-cadherin or catenin expression and dedifferentiation, malignancy, tumor aggressivity, metastasis, or a poor survival rate in several tumor types including breast, 19,20 gastric, 21,22 liver, 23 bladder, 24 prostate, 25 lung, 26 and colon 27 carcinomas. However, in some other cases, the lack of cadherin-catenin complex expression could not be correlated to any histopathological criteria of epithelial carcinomas. 9,28
To investigate the involvement of E-caherin-catenin complex in the pathophysiology of human bronchopulmonary carcinomas, we performed immunolocalization studies of E-cadherin, α-catenin, and β-catenin on several primary tumors and compared their in vivo pattern to in vitro results on four human bronchial cell lines with different invasive capacities. This study was completed by an E-cadherin immunoprecipitation experiment to check the integrity and the tyrosine phosphorylation state of the E-cadherin-catenin complex in tumors as compared to nontumoral control lung parenchyma.
Materials and Methods
Clinical Samples
Fresh tissue samples were obtained from 44 lungs resected for primary tumors including 26 squamous cell carcinomas (9 stage I, 6 stage II, 11 stage IIIα), 6 adenocarcinomas (3 stage I, 3 stage IIIα), 4 bronchioloalveolar carcinomas (4 stage I), 4 neuroendocrine tumors (1 stage I, 2 stage II, 1 stage IIIα), 2 large cell carcinomas (2 stage IIIα), and 1 carcinoid (stage II) and 1 metastasis from mammary carcinoma. Tumors were histologically classified according to the World Health Organization classification and staged according to the TNM classification. Nonneoplastic pulmonary parenchyma counterparts taken from sites adjacent to the tumor were also used for immunoprecipitation study.
Bronchial Cell Lines
The human bronchial cell lines used in this study, 16HBE14o, Beas2B, BZR, and BZR-T33, display different invasive potential in vitro and tumorigenicity and metastatic ability in athymic nude mice. 29-31 16HBE14o and Beas2B were derived from normal human bronchial cells immortalized after transfection with SV40 large T-antigen gene. BZR cell line was established from Beas2B cells by transfection with v-Ha-ras oncogene, while the BZR-T33 cell line derived from a tumor formed by BZR cells injected subcutaneously into an athymic nude mouse. 29,30 The cells were cultured at 37°C and 5% CO2 in Dulbecco modified Eagle’s medium (DMEM) supplemented with penicillin, streptomycin, ascorbic acid (50 ng/ml), and 10% fetal calf serum (Gibco BRL, Grand Island, NY).
Antibodies
The antibodies used were mouse monoclonal anti-human E-cadherin-1 (dilutions of 1/200 and 1/250 for immunohistochemistry and Western blotting, respectively) (R&D Systems, Abingdon, UK), anti-human α-catenin (dilution of 1/200 for immunohistochemistry and Western blotting) (Camfolio/Becton Dickinson, San Jose, CA), anti-human β-catenin (dilutions of 1/500 and 1/1000 for immunohistochemistry and Western blotting, respectively) (Transduction Laboratories, Lexington, KY) and anti-phosphotyrosine (PY20) (dilution of 1/250 for Western blotting) (Transduction Laboratories).
Immunohistochemistry
Tissue cryosections 5 μm thick were rehydrated in phosphate-buffered saline (PBS) and nonspecific binding was blocked with 3% bovine serum albumin-PBS for 30 minutes. Slides were incubated for 1 hour with anti-E-cadherin, anti-α-catenin, or anti-β-catenin antibodies. Negative controls were carried out by replacing the primary antibody with nonimmune IgG. After three 5-minute washes in PBS, tissue sections were treated with a biotinylated secondary antibody for 1 hour (1/50) (goat anti-mouse antibody) (Amersham, Aylesbury, UK), followed by streptavidin fluorescein complex (30 minutes, 1/50) (Amersham). All slides were counterstained with Mayer’s hematoxylin, mounted in Citifluor (UKC Chemistry Lab, Canterbury, UK) and examined under a Zeiss Axiophot microscope.
Human bronchial cells grown on four-well chamber slides (Lab-Tek, Nunc, Naperville, IL) were fixed for 10 minutes with −20°C methanol before they were subjected to immunostaining as described above.
Staining was recorded as strong (+++) when all tumor cells showed reactivity, as moderate (++) and faint (+) when reactivity was lacking in a fraction of tumor cells (50 to 90% and 10 to 49%, respectively), and as negative (−) when there was no reactivity. Localization of the staining (membranous and cytoplasmic pattern) was also evaluated.
Immunolocalizations of E-cadherin and catenins were also observed using an MRC 600 Biorad confocal laser scanning microscope, which allows the observation of 0.2-μm-thick optical sections.
Immunoprecipitation
Proteins in lung samples were extracted in 1 ml of lysis buffer (1% Triton X-100, 1% Nonidet P-40, 0.2 mmol/L leupeptin, 10 mmol/L Pefablock, 2.77 nmol/L aprotinin, 10 mmol/L NaF and 1 mmol/L NaVO3 in PBS) per 20 mg of tissue. Lysates were cleared by spinning at 12,500 × g for 10 minutes. Protein concentrations were determined with the BCA protein assay reagent (Pierce, Rockford, IL). Five hundred μg of each protein sample were preincubated with protein G-Sepharose CL-4B beads (Pharmacia Biotech AB, Uppsala, Sweden), by rocking 1 hour at 4°C. These beads were discarded and the supernatants were incubated with either 1 μg of human E-cadherin-1 or 4 μg of PY20 antibody for 3 hours on a rotating wheel at 4°C. Protein G-Sepharose beads were then added and the samples incubated for 1 hour at 4°C. Immunoprecipitates were washed six times in lysis buffer and boiled in 30 μl of Laemmli sample buffer before they were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions.
Western Blotting
Proteins from human bronchial cell lines were solubilized in Laemmli sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5% phastgels), and subsequently transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA) using the Phast system (Pharmacia Biotech AB).
For tissue samples, immunoprecipitates were separated on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under reducing conditions and transferred to immobilon polyvinylidene difluoride membrane (Millipore) using the Biorad miniprotean II electrophoresis and the mini-trans-blot electrophoretic systems, respectively (Biorad, Hercules, CA). To visualize protein transfer, blots were reversibly stained with 0.2% Ponceau-S in 3% trichloroacetic acid. All subsequent incubations were done on a rotary platform. Blots were treated for 1 hour in blocking buffer (5% low-fat milk powder, 0.1% Tween-20 in PBS), then incubated with primary antibodies in blocking buffer overnight at 4°C. Next, biotin-conjugated second antibodies were applied, followed by streptavidin-horseradish peroxidase in blocking buffer and in PBT (0.1% Tween-20 in PBS), respectively. After each incubation, blots were washed three times with blocking buffer and also twice in PBT before the ECL detection (Amersham, Buckinghamshire, UK).
Statistical Analyses
Statistical analyses of E-cadherin and catenins expression patterns were made using the χ 2 test (with Yates’ correction for adjustment of the continuity of the χ 2 distribution when necessary). Differences between two populations were considered significant when confidence intervals were >95% (P < 0.05).
Results
Study of Bronchial Cell Lines
Immunohistochemistry
By immunohistochemistry, adhesion molecule expression correlated with the cell morphology. Indeed, whereas noninfiltrating 16HBE cells, which displayed a polygonal epithelial phenotype, expressed E-cadherin, α-catenin and β-catenin, the moderately infiltrating Beas2B cell line and the highly infiltrating BZR and BZR-T33 cell lines, all displaying an elongated fibroblastoid shape, did not express E-cadherin or α-catenin (data not shown). Only β-catenin was always detected in all cell lines. The spatial distribution study by confocal microscopy revealed a strong membranous E-cadherin, α-catenin, and β-catenin expression pattern in 16HBE cells (Figure 1A ▶ −C). On the contrary, β-catenin was distributed in a spotty cytoplasmic pattern in Beas2B, BZR, and BZR-T33 invasive cell lines (Figure 1D) ▶ .
Figure 1.
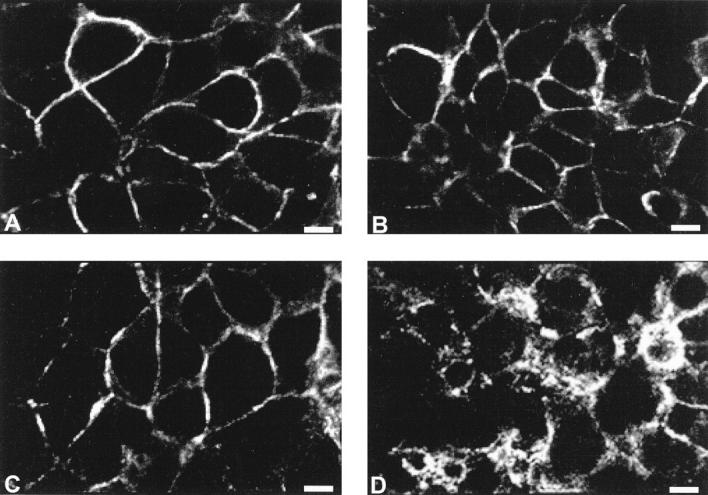
Spatial distribution of E-cadherin and catenins in human bronchial cell lines by confocal microscopy. Ephithelioid noninvasive 16HBE cells displayed a strong membranous E-cadherin (A), α-catenin (B), and β-catenin (C) expression pattern. However, only β-catenin was expressed in highly invasive BZR cells, where it was distributed in a spotty cytoplasmic pattern (D). Scale bar = 11 μm.
Western Blot Analysis
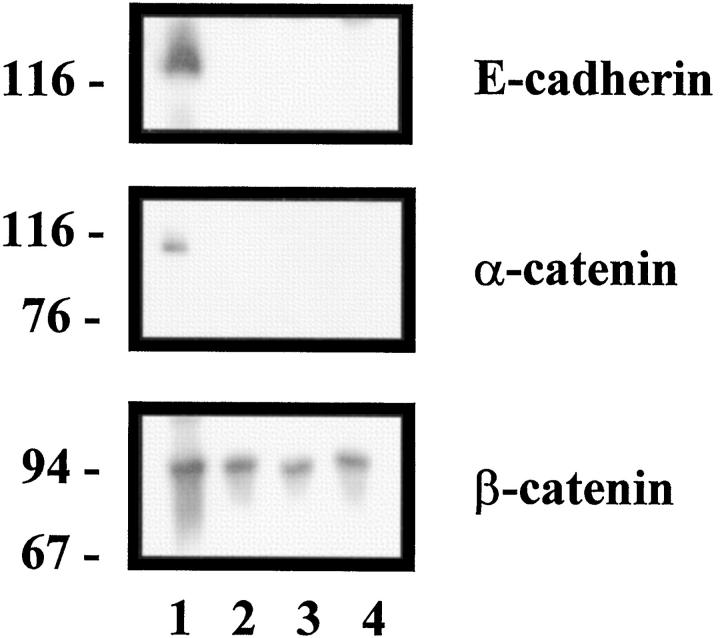
The immunohistochemical results were confirmed by a Western blot study. Only the noninvasive 16HBE cell line expressed E-cadherin, α-catenin, and β-catenin. In invasive cell lines, only β-catenin expression persisted in invasive cell lines (Figure 2) ▶ .
Figure 2.
Detection of E-cadherin and catenins expression in human bronchial cell lines by Western blot analysis. 16HBE cells expressed E-cadherin, α-catenin and β-catenin (lane 1), whereas Beas2B (lane 2), BZR (lane 3), and BZR-T33 (lane 4) cells expressed only β-catenin.
In Vivo Study
Immunolocalization
These results are summarized in Table 1 ▶ .
Table 1.
Immunolocalization Results for 44 Tumor Tissue Samples
| Histological type* | Differentiation state† | TNM stage | Labeling intensity‡ | Distribution pattern§ |
|---|---|---|---|---|
| A | W | I | ++ | mb |
| A | W | I | ++ | cy |
| A | M | I | ++ | mb |
| A | W | III | +++ | mb |
| A | M | III | ++ | mb |
| A | P | III | ++ | mb |
| S | W | I | +++ | cy |
| S | W | I | +++ | cy |
| S | M | I | ++ | cy |
| S | M | I | ++ | cy |
| S | P | I | ++ | mb |
| S | P | I | + | cy |
| S | P | I | ++ | cy |
| S | P | I | ++ | cy |
| S | P | I | + | cy |
| S | M | II | ++ | mb |
| S | M | II | + | cy |
| S | M | II | ++ | cy |
| S | M | II | ++ | cy |
| S | M | II | ++ | cy |
| S | P | II | − | |
| S | M | III | + | cy |
| S | M | III | ++ | cy |
| S | M | III | ++ | cy |
| S | M | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | ++ | cy |
| S | P | III | +++ | cy |
| BA | W | I | ++ | mb |
| BA | W | I | +++ | mb |
| BA | W | I | +++ | mb |
| BA | W | I | +++ | cy |
| NE | I | + | cy | |
| NE | II | ++ | cy | |
| NE | M | II | ++ | cy |
| NE | III | +++ | cy | |
| LC | III | − | ||
| LC | III | + | cy | |
| C | W | II | ++ | cy |
| ME | ++ | cy |
A, adenocarcinoma; S, squamous cell carcinoma; C, carcinoid mammary tumor; NE, neuroendocrine tumor; BA, bronchioalveolar carcinoma; LC, large cell carcinoma; ME, metastasis.
†P, poorly, M, moderately, W, well differentiated.
‡Reactivity staining grader: +++, strong; ++, moderate; +, faint; −, negative.
§mb, membrane; cy, intracytoplasmic.
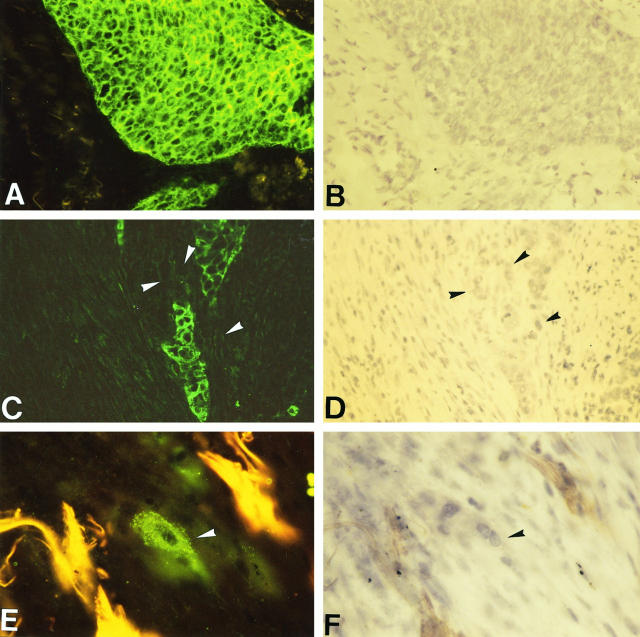
Immunohistochemistry of E-cadherin and catenins on human bronchopulmonary carcinomas revealed a very good persistence of E-cadherin and catenin expression in tumor clusters; only 2 cases were completely negative for all components of the cadherin-catenin complex. The other 42 cases were positive for E-cadherin, α-catenin, and β-catenin in nearly all tumor cells (Figure 3A and 3B) ▶ , except at the periphery of some poorly differentiated tumor clusters where positivities were heterogeneous. Moreover, E-cadherin and α-catenin were totally missing in numerous infiltrating isolated tumor cells detached from the primary tumor (Figure 3C and 3D) ▶ , whereas β-catenin expression was globally more maintained (Figure 3E and 3F) ▶ . However, the positive samples showed different intensities and distribution patterns of the adhesion complex even though labeling levels and patterns were similar for the 3 antigens in each case. Indeed, 8 cases displayed strong labeling, 28 cases moderate labeling, and 6 cases faint labeling. As for the cellular distribution investigated by confocal microscopy, 10 samples displayed a thin continuous localization of adhesion molecules along the tumor cell plasma membrane, a pattern similar to that of normal bronchial epithelium which showed a strong membranous cadherin-catenin labeling at the lateral cell borders (Figure 4, A, C, and E) ▶ . On the other hand, 32 samples had a spotty and diffuse pattern corresponding to a clear intracytoplasmic distribution of E-cadherin and catenins. This pattern was observed in most invasive tumor nests infiltrating the stroma and never found in preinvasive lesions in situ (Figure 4, B, D, and F) ▶ . Statistically, the labeling intensity was not correlated to the labeling pattern; a tumor with an internalized pattern did not necessarily have faint labeling. On the other hand, labeling intensity was not related to tumor histological type nor to tumor TNM stage, whereas it was significantly correlated to the differentiation state. Indeed, only 4% of moderately or poorly differentiated tumors displayed a strong labeling versus 60% of well-differentiated tumors (P = 0.0006). Moreover, the cellular distribution pattern was correlated to the histological type; 80% of adenocarcinomas including bronchioloalveolar carcinomas showed a normal membranous E-cadherin and catenin distribution at the cell membrane versus 8% of squamous cell carcinomas (P = 0.0001). However, the cellular distribution pattern was not related to the differentiation state of squamous cell carcinomas or to the tumor TNM stage.
Figure 3.
Localization by immunofluorescence of E-cadherin-catenin complex molecules on cryosections of bronchopulmonary carcinomas. E-cadherin was strongly expressed in tumor cells of a squamous cell carcinoma (A); hematoxylin counterstaining (B). Magnification, ×160. Some infiltrating isolated tumor cells (arrowheads) that were detached from the primary tumor were E-cadherin-negative (C); heamatoxylin counterstaining (D). Magnification, ×160. Isolated invasive tumor cells (arrowhead) preserved a cytoplasmic β-catenin expression (E); hematoxylin counterstaining (F). Magnification, ×320.
Figure 4.
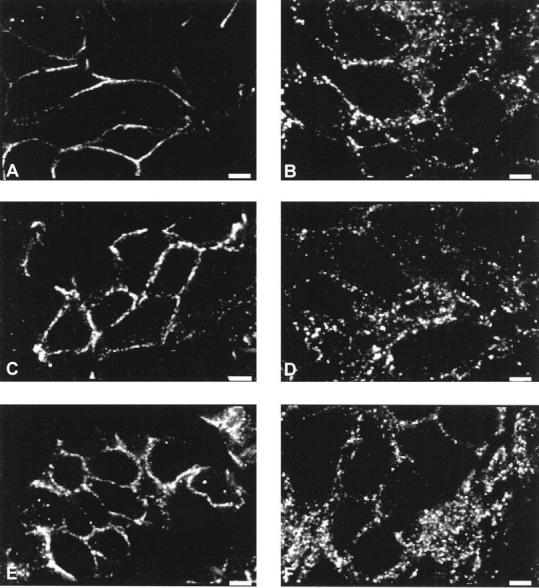
Spatial distribution of E-cadherin and catenins in bronchopulmonary carcinomas by confocal microscopy. E-cadherin (A), α-catenin (C), and β-catenin (E) were expressed in a thin pericellular pattern in an adenocarcinoma. A focal area of invasive tumor cluster of a squamous cell carcinoma displayed a clear intracytoplasmic distribution of E-cadherin (B), α-catenin (D), and β-catenin (F). Scale bar = 6 μm.
Immunoprecipitation of Cadherin-Catenin Complex Study
Because in vivo invasion of bronchopulmonary tumor cells was not related to a real loss of cadherin and catenin expression, but rather to a redistribution of the components of this complex, we performed an E-cadherin immunoprecipitation study to investigate the integrity of the cadherin-catenin complexes. This study was performed on 8 nontumoral lung parenchyma samples, 2 carcinomas with a membranous pattern (1 adenocarcinoma and 1 squamous cell carcinoma) and 5 carcinomas with a spotty cytoplasmic pattern (2 adenocarcinomas and 3 squamous cell carcinomas).
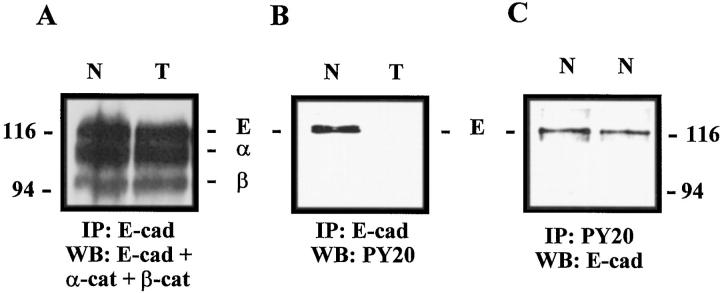
E-cadherin immunoprecipitation followed by Western blot analysis indicated that E-cadherin was complexed to β- and α-catenins in all samples tested (nontumoral parenchyma, carcinomas with a membranous pattern, and carcinomas with a spotty cytoplasmic distribution) (Figure 5A) ▶ . Then the tyrosine phosphorylation state of the cadherin-catenin complex was analyzed. Whereas E-cadherin was tyrosine-phosphorylated in the 2 nontumoral samples and in the 2 carcinomas showing a membranous pattern, its tyrosine phosphorylation was down-regulated in the 5 carcinomas displaying a spotty cytoplasmic pattern (Figure 5B) ▶ . No α- and β-catenin tyrosine phosphorylation was observed in either the carcinomas or the nontumoral samples. Immunoprecipitation with antiphosphotyrosine PY20 antibody confirmed that the tyrosine-phosphorylated band was E-cadherin in nontumoral samples (Figure 5C) ▶ .
Figure 5.
E-cadherin immunoprecipitation in nontumoral and tumoral samples. A: E-cadherin was complexed to α- and β-catenins in nontumoral lung parenchyma (N) and in a tumoral sample displaying a cytoplasmic pattern (T). B: E-cadherin was tyrosine-phosphorylated in the same nontumoral sample (N), whereas no tyrosine phosphorylation was detected in the carcinoma sample (T). C: Immunoprecipitation by PY20 antibody confirmed that tyrosine-phosphorylated band was E-cadherin in normal samples (N).
Discussion
The tumor suppressor function of E-cadherin has been well documented in many in vitro studies showing down-regulation of E-cadherin and/or α-catenin in invasive cancer cell lines. 31-34 This is in agreement with our results showing a total lack of E-cadherin and α-catenin expression in the invasive bronchial cell lines studied. In the same way, the loss of adhesion molecules was confirmed in vivo in most isolated infiltrating tumor cells detached from the primary tumor clusters, underlining tumor heterogeneity. These E-cadherin and α-catenin negative cells in vivo may represent the most aggressive and potentially metastatic tumor cell contingent that has been selected in vitro in our cell lines. Thus, the total loss of E-cadherin and/or α-catenin may be a late event in bronchopulmonary carcinomas. Concerning the tumor clusters, particularly those which were well differentiated, E-cadherin, α-catenin, and β-catenin remained globally highly expressed. In agreement with observations of Böhm et al, 26 we found a significant correlation between decreased labeling intensity of adhesion molecules and the loss of differentiation in tumor nests of bronchopulmonary carcinomas. E-cadherin has already been considered as a cellular marker of tumor dedifferentiation confirmed for gastric, 21,22 hepatocellular, 23 bladder, 24 prostate, 25 and esophageal carcinomas. 35 However, we did not find any correlation between decreased E-cadherin and catenin immunoreactivity and tumor TNM stage. Depending on the type of cancers studied and the detection methods used, such a correlation between negative regulation of adhesion molecule expression and tumor progression is not always found in vivo. 9,20,36 Numerous immunohistochemical studies have been performed on formalin-fixed paraffin-embedded tissue sections with a less sensitive detection than frozen sections. 8,22,24,25,35,37 In some tumor cells located in clusters, we observed a redistribution of the E-cadherin complex molecules from the cell surface to the cytoplasm. Similar results for E-cadherin expression in lung carcinomas were also obtained in two other studies. 26,28 This redistribution appears to be related to the histological type of carcinomas because adenocarcinomas generally preserved a membranous pattern of expression, whereas a spotty cytoplasmic pattern was observed in squamous cell carcinomas. We could not establish any significant correlation between cytoplasmic redistribution of the E-cadherin complex molecules and tumor grade. Nevertheless, the cytoplasmic redistribution was observed particularly in restricted invasive nests and never in in situ lesions, suggesting that such a redistribution, altering cell-cell contacts, could cause specific tumor cells to become invasive. Considering that total loss of E-cadherin and catenins expression contributes to the invasive behavior of isolated invasive tumor cells, the present data suggest that another mechanism would be used by tumor cells which have conserved the expression of these molecules and which are located in cohesive tumor clusters. The three different patterns of E-cadherin complex molecule expression (ie, membranous, spotty patterns, and loss of expression) could represent sequential stages toward the invasive phenotype.
The mechanisms by which the molecules of the E-cadherin complex are redistributed remain elusive. We performed immunoprecipitation experiments to address this point and to reveal possible modifications in the interactions between E-cadherin and the catenins in the diverse patterns of expression described above. We found no difference between nontumoral and tumoral tissues as regards the link between catenins and E-cadherin. Indeed, we observed the preservation of adhesion complex integrity in all of the primary tumors tested. However, regarding phosphorylation state, we found a difference of phosphorylation on tyrosine residues of E-cadherin between nontumoral samples and tumors with different expression patterns. Whereas E-cadherin tyrosine phosphorylation was detected in nontumoral lung parenchyma and in carcinomas showing a well preserved membranous pattern, a down-regulated tyrosine phosphorylation was observed in primary tumors displaying a spotty cytoplasmic pattern. Thus, the adhesion complex redistribution may be related to E-cadherin dephosphorylation. A previous study performed on thyroid carcinomas also pointed to the absence of E-cadherin tyrosine phosphorylation in carcinomas as compared to nonmalignant tissues and this state was related to a pericellular redistribution of the complex with no synthesis variations for E-cadherin or catenins. 38 The authors suggested that the cadherin-catenin complex was disconnected from the cytoskeleton. 38 Other studies have also shown involvement of phosphorylation/dephosphorylation of the cadherin-catenin complex with respect to the regulation of its cellular adhesion function. The role of tyrosine phosphorylation of cadherin-catenin complex and in particular of β-catenin is not clear because catenin-E-cadherin interaction is generally not affected. Instead of a disruption of the complex, modifications in tyrosine phosphorylation of adhesion molecules may induce the dissociation of E-cadherin and catenins from the cytoskeleton and thus dispersion of the complexes in the cell. 6,12,13,36,39 Therefore we could speculate that, in the case of bronchopulmonary carcinomas, redistribution of the cadherin-catenin complex in association with a decreased tyrosine phosphorylation of E-cadherin may reflect a loss of adhesion functionality leading to the acquisition of an invasive phenotype.
In this study we have also observed that, in contrast with the other members of the complex, β-catenin expression persisted in invasive bronchial cell lines and in most infiltrating tumor cells of bronchopulmonary carcinomas; its distribution pattern was, however, dramatically modified. The membranous pattern was replaced by a spotty and cytoplasmic pattern in invasive cancer cells. This observation emphasizes the multiple roles of this protein. Indeed, β-catenin has been shown to possess a cellular signaling capacity as a participant in the developmental Wnt-signal transduction pathway. Indeed, cytoplasmic β-catenin is involved in cell migration process via interaction with the product of adenomatous polyposis coli tumor suppressor gene and cytoskeleton. 40-46 On the other hand, free cytoplasmic β-catenin plays a role in activation of some genes, in particular cell proliferation-stimulating genes, or apoptosis-antagonizing genes and mesenchymal genes, by complexation with DNA-binding transcription factors such as lymphoid enhancer-binding factor (LEF)-1. 40,42,47-49 Moreover, it has been shown that the LEF-1-β-catenin complex binds in vitro to the E-cadherin promoter to regulate the transcription of this gene, suggesting that although adhesion function and intracellular signaling activity of β-catenin are independent, they could be interrelated via negative regulation by E-cadherin. 1,40,45,50 This is in agreement with our results showing that bronchial invasive cell lines and most in vivo isolated tumor cells displaying a spotty and cytoplasmic β-catenin labeling were E-cadherin-negative. Globally, this observation suggests that the cytoplasmic distribution of β-catenin may also reflect the invasive potential of cells.
In conclusion, our results show that the total loss of cadherin-catenin complex expression is a rare event in human bronchopulmonary carcinomas, restricted to highly infiltrative tumor cells. The most prominent observation is a cytoplasmic redistribution of the E-cadherin complex molecules associated with a decreased tyrosine phosphorylation of E-cadherin, suggesting that, at least in vivo, this characteristic may be a prerequisite for tumor cells to acquire an invasive pattern in lung tissue.
Acknowledgments
We thank Dr. C.C. Harris (National Institutes of Health, Bethesda, MD) for providing Beas2B, BZR, and BZR-T33 cell lines, Dr. D. Gruenert (University of California, San Francisco, CA,) for providing 16HBE cell line, and Dr. M. Monteau (Polyclinique Courlancy, Reims, France) for providing lung tissues.
Footnotes
Address reprint requests to Béatrice Nawrocki, INSERM U.314, Hôpital Maison Blanche, 45, rue Cognacq-Jay, 51100 Reims, France. E-mail: birembau@centre02-univ-reims.fr.
Supported in part by the Lions Club of Soissons, by a grant from La Ligue contre le cancer (département de la Marne), and by ASLK-VIVA (Belgium).
References
- 1.Huber O, Bierkamp C, Kemler R: Cadherins and catenins in development. Curr Opin Cell Biol 1996, 8:685-691 [DOI] [PubMed] [Google Scholar]
- 2.Pignatelli M, Vessey CJ: Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol 1994, 25:849-856 [DOI] [PubMed] [Google Scholar]
- 3.Elangbam CS, Qualls CW, Jr, Dahlgren RR: Cell adhesion molecules: update. Vet Pathol 1997, 34:61-73 [DOI] [PubMed] [Google Scholar]
- 4.Albelda SM: Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 1993, 68:4-17 [PubMed] [Google Scholar]
- 5.Munro SB, Blaschuk OW: The structure, function and regulation of cadherins. Brodt P eds. In Cell Adhesion and Invasion in Cancer Metastasis. 1996, :pp 17-34 RG Landes Company, Springer, New York [Google Scholar]
- 6.Aberle H, Schwartz H, Kemler R: Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 1996, 61:514-523 [DOI] [PubMed] [Google Scholar]
- 7.Mareel M, Berx G, van Roy F, Bracke M: Cadherin/catenin complex: a target for antiinvasive therapy? J Cell Biochem 1996, 61:524-530 [DOI] [PubMed] [Google Scholar]
- 8.Zschiesche W, Schönborn I, Behrens J, Herrenknecht K, Hartveit F, Lilleng P, Birchmeier W: Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res 1997, 17:561-568 [PubMed] [Google Scholar]
- 9.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 10.Simard D, Nabi IR: Inverse relation of autocrine motility factor receptor and E-cadherin expression following MDCK epithelial cell transformation. Biochem Biophys Res Commun 1996, 219:122-127 [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen SJ, Bruyneel EA, Bracke ME, de Bruyne GK, Vennekens KM, Vlemincks KL, Berx GJ, van Roy FM, Mareel M: Transition from the noninvasive to the invasive phenotype and loss of α-catenin in human colon cancer cells. Cancer Res 1995, 55:4722-4728 [PubMed] [Google Scholar]
- 12.Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S: Dominant negative inhibition of the association between β-catenin and c-erbB-2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene 1996, 13:883-889 [PubMed] [Google Scholar]
- 13.Behrens J, Vakaet L, Friis R, Winterhager E, van Roy F, Mareel M, Birchmeier W: Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 1993, 120:757-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vleminckx K, Vakaet L, Mareel M, Fiers W, van Roy F: Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991, 66:107-119 [DOI] [PubMed] [Google Scholar]
- 15.Ewing CM, Ru N, Morton RA, Robinson JC, Wheelock MJ, Johnson KR, Barrett JC, Isaacs WB: Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of α-catenin and restoration of E-cadherin function. Cancer Res 1995, 55:4813-4817 [PubMed] [Google Scholar]
- 16.Wilding J, Vousden KH, Soutter WP, McCrea PD, del Buono R, Pignatelli M: E-cadherin transfection down-regulates the epidermal growth factor receptor and reverses the invasive phenotype of human papilloma virus-transfected keratinocytes. Cancer Res 1996, 56:5285-5292 [PubMed] [Google Scholar]
- 17.Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T: E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res 1996, 56:4063-4070 [PubMed] [Google Scholar]
- 18.Miyaki M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Konishi M, Takeichi M: Increased cell-substratum adhesion, and decreased gelatinase secretion and cell growth, induced by E-cadherin transfection of human colon carcinoma cells. Oncogene 1995, 11:2547-2552 [PubMed] [Google Scholar]
- 19.Guriec N, Marcellin L, Gairard B, Calderoli H, Wilk A, Renaud R, Bergerat JP, Oberling F: E-cadherin mRNA expression in breast carcinomas correlates with overall and disease-free survival. Invasion Metastasis 1996, 16:19-26 [PubMed] [Google Scholar]
- 20.Hashizume R, Koizumi H, Ihara A, Ohta T, Uchikoshi T: Expression of β-catenin in normal breast tissue and breast carcinoma: a comparative study with epithelial cadherin and α-catenin. Histopathology 1996, 29:139-146 [DOI] [PubMed] [Google Scholar]
- 21.Matsuura K, Kawanishi J, Fujii S, Imamura M, Hirano S, Takeichi M, Niitsu Y: Altered expression of E-cadherin in gastric cancer tissues and carcinomatous fluid. Br J Cancer 1992, 66:1122-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G: Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer (Pred Oncol) 1996, 69:184-189 [DOI] [PubMed] [Google Scholar]
- 23.Kozyraki R, Scoazec JY, Flejou JF, d’Errico A, Bedossa P, Terris B, Fiorentino M, Bringuier AF, Grigioni WF, Feldmann G: Expression of cadherins and α-catenin in primary epithelial tumors of the liver. Gastroenterology 1996, 110:1137-1149 [DOI] [PubMed] [Google Scholar]
- 24.Syrigos KN, Krausz T, Waxman J, Pandha H, Rowlinson-Busza G, Verne J, Epenetos AA, Pignatelli M: E-cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer (Pred Oncol) 1995, 64:367-370 [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG: Expression of E-cadherin in primary and metastatic prostate cancer. Am J Pathol 1996, 148:1375-1380 [PMC free article] [PubMed] [Google Scholar]
- 26.Böhm M, Totzeck B, Birchmeier W, Wieland I: Differences of E-cadherin expression levels and patterns in primary and metastatic lung cancer. Clin Exp Metastasis 1994, 12:55-62 [DOI] [PubMed] [Google Scholar]
- 27.Dorudi S, Hanby AM, Poulsom R, Northover J, Hart IR: Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer 1995, 71:614-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han AC, Peralta-Soler A, Knudsen KA, Wheelock MJ, Johnson KR, Salazar H: Differential expression of N-cadherin in pleural mesotheliomas and E-cadherin in lung adenocarcinomas in formalin-fixed, paraffin-embedded tissues. Hum Pathol 1997, 28:641-645 [DOI] [PubMed] [Google Scholar]
- 29.Ura H, Bonfil D, Reich R, Reddel R, Pfeifer A, Harris C, Klein-Szanto A: Expression of type IV collagenase and procollagen genes and its correlation with the tumorigenic, invasive, and metastatic abilities of oncogene-transformed human bronchial epithelial cells. Cancer Res 1989, 49:4615-4621 [PubMed] [Google Scholar]
- 30.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC: CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 1994, 10:38-47 [DOI] [PubMed] [Google Scholar]
- 31.Polette M, Gilles C, de Bentzmann S, Gruenert D, Tournier JM, Birembaut P: Association of fibroblastoid features with the invasive phenotype in human bronchial cancer cell lines. Clin Exp Metastasis 1998, 16:105-112 [DOI] [PubMed] [Google Scholar]
- 32.Oda T, Kanai Y, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S: Cloning of human α-catenin cDNA and its aberrant mRNA in a human cancer cell line. Biochem Biophys Res Commun 1993, 193:897-904 [DOI] [PubMed] [Google Scholar]
- 33.Sommers CL, Thompson EW, Torri J, Kemler EP, Gelmann EP, Byers SW: Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ 1991, 2:365-372 [PubMed] [Google Scholar]
- 34.Gilles C, Polette M, Birembaut P, Brunner N, Thompson EW: Expression of c-est-1 mRNA is associated with an invasive, EMT-derived phenotype in breast carcinoma cell lines. Clin Exp Metastasis 1997, 154:519-526 [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S: Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance. Oncology 1997, 54:158-165 [DOI] [PubMed] [Google Scholar]
- 36.Boyer B, Vallés AM, Thiery JP: Model systems of epithelium-mesenchyme transitions. Acta Anat 1996, 156:227-239 [DOI] [PubMed] [Google Scholar]
- 37.Yagasachi R, Noguchi M, Minami M, Earashi M: Clinical significance of E-cadherin and vimentin co-expression in breast cancer. Int J Oncol 1996, 9:755-761 [DOI] [PubMed] [Google Scholar]
- 38.Serini G, Trusolino L, Saggiorato E, Cremona O, de Rossi M, Angeli A, Orlandi F, Marchisio PC: Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J Natl Cancer Inst 1996, 88:442-449 [DOI] [PubMed] [Google Scholar]
- 39.Sommers CL, Gelmann EP, Kemler R, Cowin P, Byers W: Alterations in β-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res 1994, 54:3544-3552 [PubMed] [Google Scholar]
- 40.Miller JR, Moon RT: Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev 1996, 10:2527-2539 [DOI] [PubMed] [Google Scholar]
- 41.Pollack AL, Barth AIM, Altschuler Y, Nelson WJ, Mostov KE: Dynamics of β-catenin interactions with APC protein regulate epithelial tubulogenesis. J Cell Biol 1997, 137:1651-1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peifer M: β-catenin as oncogene: the smoking gun. Science 1997, 275:1752-1753 [DOI] [PubMed] [Google Scholar]
- 43.Vleminckx K, Wong E, Guger K, Rubinfeld B, Polakis P, Gumbiner BM: Adenomatous polyposis coli tumor suppressor protein has signaling activity in Xenopus laevi embryos resulting in the induction of an ectopic dorsoanterior axis. J Cell Biol 1997, 136:411-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funayama N, Fagotto F, mc Crea P, Gumbiner BM: Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol 1995, 128:959-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumbiner BM: Signal transduction by β-catenin. Curr Opin Cell Biol 1995, 7:634-640 [DOI] [PubMed] [Google Scholar]
- 46.Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, mc Crea PD: β-catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol 1996, 134:1271-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P: Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272:1023-1026 [DOI] [PubMed] [Google Scholar]
- 48.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P: Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 1995, 92:3046-3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behrens J, von Kries JP, Kühl M, Bruhm L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 50.Fagotto F, Funayama N, Glück U, Gumbiner BM: Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in xenopus. J Cell Biol 1996, 132:1105-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]