Abstract
The expression of the natural killer (NK) cell marker CD56 has been reported to occur in NK cell lymphomas/leukemias and a small group of peripheral T-cell lymphomas but has not been studied extensively in primary intestinal non-B-cell lymphomas. Normal human jejunal intraepithelial lymphocytes (IELs) are mainly T-cell receptor (TCR)-αβ+CD3+CD8+CD5low and include an ∼15% fraction of CD56+ cells that could be the cells of origin for CD56+ intestinal T-cell lymphoma (ITL). To test this hypothesis, 70 cases diagnosed as ITL were immunophenotyped, and 15 CD56+ cases (21%) were identified. The majority of the CD56+ lymphomas was of monomorphic small to medium-sized histology, shared the common phenotype βF1±CD3ε/cyt+CD8+CD4−CD5−CD57−TIA-1+ and had clonally rearranged TCR γ-chain genes. In contrast, the CD56− lymphomas were mainly composed of pleomorphic medium and large cells or had a morphology most consistent with anaplastic large-cell lymphoma and were mostly CD8−. These findings suggest that the majority of CD56+ intestinal lymphomas are morphologically and phenotypically distinct T-cell lymphomas most likely derived from activated cytotoxic CD56+CD8+ IELs. Some overlapping histological and clinical features between CD56+ and CD56− ITLs indicate that the former belong to the clinicopathological entity of ITL. The consistent expression of cytotoxic-granule-associated proteins introduces ITL (both CD56+ and CD56−) into the growing family of usually aggressive extranodal lymphomas of cytotoxic T-cell and NK-cell derivation. In contrast to putative NK-cell lymphoma of the sinonasal region, intestinal NK-cell lymphoma seems to be very rare.
In 1978, Isaacson and Wright reported 18 patients with small-intestinal lymphoma associated with villous atrophy and crypt hyperplasia of uninvolved mucosa. 1 Malabsorption, thought to be adult celiac disease (CD), preceded the diagnosis of lymphoma in five of these patients. The authors concluded that the tumors were of a single histogenetic type, probably derived from histiocytes, and made the diagnosis of malignant histiocytosis. Subsequent studies showed that the tumor cells had clonally rearranged T cell receptor (TCR) genes and expressed T-cell-associated antigens, consistent with T-cell derivation. 2-4 The coincidence of intestinal T-cell lymphoma (ITL) with villous atrophy of the jejunal mucosa prompted O’Farrelly and co-workers to coin the term enteropathy-associated T-cell lymphoma (EATCL), which has been widely used since then. 5 Comparison between the immunological phenotypes of normal intestinal intraepithelial lymphocytes (IELs) and ITLs provided some evidence that ITL might be the neoplastic counterpart of IELs. 4,6-8
The expression of the natural killer (NK) cell marker CD56 has been reported to occur in NK-cell lymphomas/leukemias and a small group of peripheral T-cell lymphomas 9-11 but has not been studied extensively on primary intestinal non-B-cell lymphomas. The present study on 70 cases diagnosed as ITL identified 15 CD56+ lymphomas (21%) and showed that the majority of these neoplasms are not only immunophenotypically distinct peripheral T-cell lymphomas but do also share characteristic histological features.
Materials and Methods
Tissue
A total of 70 cases diagnosed as ITL were retrieved from the files of the Department of Clinical Pathology, General Hospital Vienna (n = 42) and from the Department of Pathology, University of Würzburg (n = 28). From our previously published series of 27 cases, 8 22 ITLs were included in this study, the remaining 5 cases were excluded because only endoscopic biopsies were available (n = 2) or too little tissue was left in the blocks (n = 3). Surgical resection specimens were available in all cases for detailed histopathological and immunophenotypical analyses. Frozen tissue was available in 13 cases.
Histological Definition of Enteropathy
The intestinal mucosa uninvolved by lymphoma was evaluated for the presence of enteropathy with special emphasis on an increase in normal-appearing IELs. Although in patients with untreated CD, an increase in IELs is usually accompanied by architectural changes of the mucosa, such as crypt hypertrophy and/or variable degrees of villous atrophy, the increase in IELs is of special importance as this feature represents the earliest morphological finding and is observed in virtually all patients, including subjects with latent CD and even in family relatives. 12 In normal controls, IELs are more frequent in jejunum than in ileum (20 ± 5 versus 9 ± 2 per 100 enterocytes). 13 For the purpose of this study, enteropathy was defined solely by an increase of IELs (≥40/100 enterocytes in jejunal and ≥20/100 enterocytes in ileal specimens) because all of the specimens that showed this feature had also at least crypt hypertrophy and/or mild villous atrophy. Conversely, absence of enteropathy was defined by IEL counts within the normal range, which was consistently found in a structurally normal mucosa.
Immunohistochemistry
Immunohistochemical analysis was performed in all 70 cases on formalin-fixed, paraffin-embedded tissue sections. Immunostaining was done using the polyclonal antibody anti-CD3 recognizing the cytoplasmic CD3-ε chain (Dako, Copenhagen, Denmark; 1:400), and the monoclonal antibodies L26 (CD20, Dako; 1:200), βF1 (T-Cell Sciences, Woburn, MA; 1:10), CD4 (Novocastra, Newcastle, UK; 1:10), CD5 (Novocastra; 1:20), CD8 (Dako; 1:30), TIA-1 (Coulter, Hialeah, FL; 1:800), CD56 (Sanbio, Uden, The Netherlands; 1:200), CD57 (Becton-Dickinson, San Jose, CA; 1:10), CD30 (Dako; 1:80), and epithelial membrane antigen (EMA) (Dako; 1:100). The monoclonal antibody granzyme B-4 (GB-4, 1:50) was generously provided by Dr. J.A. Kummer, Department of Pathology, Free University Hospital, Amsterdam, The Netherlands. Pretreatment for unmasking of antigens was done either by digestion with 0.05% preheated protease (type XXIV, Sigma Chemical Co., St. Louis, MO) in Tris-buffered saline for 5 minutes at 37°C (for βF1 and CD3), or by microwaving in citrate buffer (10 mmol/L, pH 6.0) twice for 5 minutes each at 600 W (GB-4, TIA-1, CD30, and EMA) or by autoclaving at 1 bar for 20 minutes, followed by cooling down for 40 minutes (for CD4, CD5, CD8, CD56, and ALK1). Endogenous peroxidase was blocked by incubation in 1% H2O2 in Tris-buffered saline or by incubation in a solution containing glucose (50 mg/ml) and glucose oxidase (Sigma). Application of βF1, CD3, CD4, CD5, CD8, and ALK1 was followed by incubation with biotinylated goat anti-rabbit IgG (for CD3) or horse anti-mouse IgG (for βF1, CD4, CD5, CD8, and ALK1) as the secondary antibody and then by peroxidase-conjugated streptavidin (Super Sensitive HRP Label, Biogenex, San Ramon, CA). Staining was developed using 3-amino-9-ethylcarbazole as the chromogen (Sigma) in the presence of H2O2. For the remaining antibodies, biotinylated horse anti-mouse IgG (1:200) was used as the secondary antibody followed by Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine as a chromogen (Fluka, Buchs, Switzerland) in the presence of H2O2. On frozen sections, a perforin antibody (Endogen, Woburn, MA; 1:50) supplemented the antibody panel used in the previous study. 8 Nonspecific reactivity was tested by omission of the primary antibodies.
To assess the accuracy of immunostaining, all reactions were first evaluated for internal positive controls, such as small reactive lymphoid cells (for L26, βF1, CD3, CD4, CD5, CD8, GB-4, TIA-1, CD56, and CD57), histiocytes (for CD4), plasma cells (for Ber-H2 and EMA), epithelial cells (for EMA), or nerve fibers (for CD56). Staining of tumor cells was scored: +, >50% positive; ±, 20 to 50% positive; −, <20% positive; +i, individual large cells positive. The latter score was used for GB-4, Ber-H2, and EMA staining only. As an additional measure of quality control, the results obtained by frozen section immunophenotyping on 13 cases, 8 of which have been reported, 8 were compared with those on paraffin sections.
EBER in Situ Hybridization
All of the 70 cases were studied for the presence of EBV early RNA transcripts (EBERs). Fluorescein-labeled oligonucleotides complementary to EBER-1/2 were used according to the instructions of the manufacturer (PNA ISH detection kit, Dako) under RNAse-free conditions.
T-Cell Clonality Analyses by Polymerase Chain Reaction (PCR)
For the detection of T-cell clonality, a PCR technique was used to amplify rearranged TCR γ-chain gene sequences. Genomic DNA was extracted from formalin-fixed, paraffin-embedded tissue by proteinase K digestion without detergents or EDTA according to Frank and co-workers. 14 DNA was amplified in 50-μl reaction volumes essentially as described 15 with the following modifications: the primer for JGT4 was excluded from the multiplex primer mix; the primer concentrations were 10 pmol for primers V3, V4, V8, V9, V10, and V11, 15 pmol for JGT3, 20 pmol for V2 and V5, and 25 pmol for JGT12; the concentration of MgCl2 was 2.5 mmol/L, and 1.5 U of Ampli Taq Gold polymerase (Perkin Elmer Cetus, Norwalk, CT) was used per reaction. Forty-five cycles were at 94°C for 1 minute, 62°C for 1 minute, and 72°C for 1 minute with a terminal extension at 72°C for 20 minutes followed by 100°C for 6 minutes and 65°C for 3 minutes to support heteroduplex formation. PCR products were separated on precast 6% polyacrylamide gels (Novex, San Diego, CA). Simultaneously amplified DNA from a nodal peripheral T-cell lymphoma, unspecified, served as a positive control. A DNA mixture obtained from peripheral blood lymphocytes from 10 healthy donors was used as a polyclonal control.
Clinical Data
Medical records were reviewed for the patients’ history, type, duration of symptoms at initial presentation, and type of treatment. Follow-up was obtained via clinical records, attending physicians, or autopsy files. Stages of disease were assigned according to an adaptation of the Ann Arbor staging system for extranodal lymphomas and its modification by Musshoff, respectively. 16
Statistical Analyses
Differences between CD56+ and CD56− cases were analyzed using Fisher’s two-tailed exact test. Survival curves were estimated by the Kaplan-Meier method, and comparison was based on the log-rank test.
Results
Phenotypical and TCR-γ Clonality Analyses of CD56+ Intestinal Lymphomas
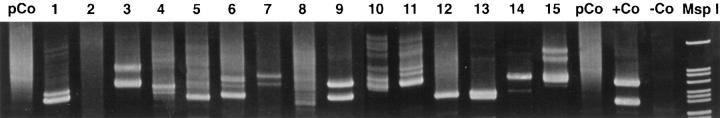
Among 70 intestinal lymphomas, which have been signed out as ITL, 15 lymphomas expressed CD56 (Table 1) ▶ . The common phenotype of the CD56+ lymphomas was βF1± (5/15), CD3ε/cyt+ (13/15), CD8+ (12/15), CD4− (15/15), CD5− (14/15), CD57− (15/15), CD30− (15/15), EMA− (15/15). Immunostaining for CD56 and CD8 in a representative case (case 13) is shown in Figure 1, A and B ▶ , respectively. The majority of CD56+ lymphomas expressed the cytotoxic-granule-associated proteins TIA-1 (14/15; Figure 1C ▶ ) and GB-4 (10/15), suggestive of cytotoxic-T-cell or NK-cell derivation. Immunohistochemistry on frozen sections in four cases showed no evidence of TCR-γδ phenotype (0/3), and in one case each, expression of perforin and CD103 (HML-1) was noted (Table 2) ▶ . To further clarify the cellular origin of the CD56+ lymphomas, TCR γ-chain gene rearrangement studies were done by multiplex PCR. Molecular evidence of clonally expanded T-cell populations was found in 12 of 15 cases (Figure 2) ▶ . No definite lineage assignment could be achieved in the three remaining lymphomas (cases 2, 8, and 10); although a T-cell origin in cases 2 and 8 could not be excluded, they more likely represented true NK-cell neoplasms; the cellular origin in case 10 (CD56+TIA-1−CD34−, myeloperoxidase−) was considered undetermined. These results suggest that the vast majority of CD56+ intestinal lymphomas are T-cell lymphomas.
Table 1.
Histological Diagnosis, Paraffin Section Immunohistochemistry, Genotyping, and EBER-ISH of 15 CD56+ Intestinal Lymphomas and Phenotypical Comparison with 55 CD56− Lymphomas
| Case | Diagnosis† | Enteropathy | βF1 | CD3ε/cyt | CD4 | CD8 | CD5 | CD56 | TIA-1 | GB-4 | TCR-γ | EBER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mono med | Yes | + | + | − | + | − | + | + | − | + | − |
| 2 | Mono med | Yes | − | + | − | + | − | + | + | − | − | − |
| 3 | Pleo m&l | No | − | + | − | + | − | + | + | − | + | − |
| 4 | Pleo m&l | Yes | + | + | − | − | + | + | + | + | + | >80% |
| 5 | IB | No | − | + | − | + | − | + | + | + | + | − |
| 6 | Mono med | No | − | + | − | + | − | + | + | + | + | − |
| 7 | Mono med | No | − | + | − | + | − | + | + | + | + | − |
| 8 | Mono med | Yes | − | − | − | + | − | + | 20% | 15% | − | − |
| 9 | Mono med | Yes | − | + | − | − | − | + | + | + | + | − |
| 10 | Pleo s | No | − | − | − | − | − | + | − | − | − | − |
| 11 | Mono s-m | Yes | + | + | − | + | − | + | + | + | + | 10–15% |
| 12 | Mono s-m | Yes | + | + | − | + | − | + | + | + | + | −† |
| 13 | Mono med | No | − | + | − | + | − | + | + | + | + | − |
| 14 | Mono s-m | Yes | + | + | − | + | − | + | + | − | + | − |
| 15 | Mono med | No | − | + | − | + | − | + | + | + | + | − |
| Total | ||||||||||||
| CD56+ | Cases | 8/15 | 5/15 | 13/15 | 0/15 | 12/15 | 1/15 | 15/15 | 13/15 | 9/15 | 12/15 | 2/15 |
| % | 53* | 33 | 87 | 0 | 80* | 7 | 100 | 87 | 60 | 80 | 13 | |
| CD56− | Cases | 44/52 | 13/53 | 50/55 | 1/53 | 10/54 | 2/54 | 0/54 | 47/55 | 45/54 | 6/6 | 3/55 |
| % | 85* | 25 | 91 | 2 | 19* | 4 | 0 | 85 | 83 | 100 | 5 |
All 70 intestinal lymphomas were negative for CD20, CD57, and ALK1. None of the 15 CD56+ lymphomas expressed Ber-H2 (CD30) or EMA. Mono med, monomorphic medium-sized cell lymphoma; Pleo m&l, pleomorphic medium and large cell lymphoma; IB, immunoblastic lymphoma; Pleo s, pleomorphic small-cell lymphoma; Mono s-m, monomorphic small- to medium-sized-cell lymphoma.
* Significant distinguishing features between CD56+ and CD56− lymphomas.
† Few scattered EBER+ cells present but indistinguishable from reactive lymphocytes.
Figure 1.
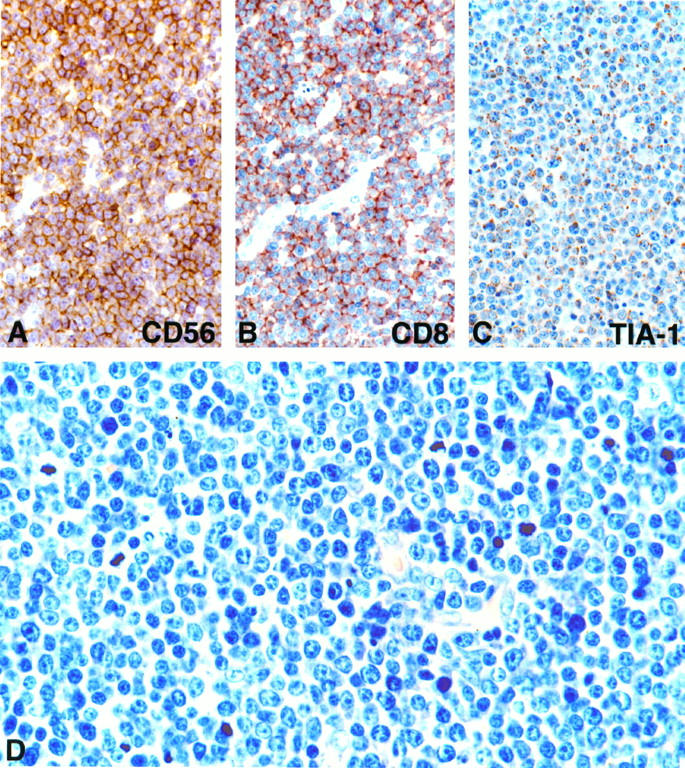
Immunophenotyping and histological appearance of representative CD56+ intestinal T-cell lymphoma (case 13). A: Lymphoma cells show strong reactivity for CD56. B: Strong staining is visible for CD8. C: Cytoplasmic granules are positive for TIA-1. D: Histology shows densely packed monomorphic medium-sized cells with round nuclei, small nucleoli, and moderately wide pale or clear cytoplasm; inflammatory cells and fibrosis are not seen. Giemsa staining; magnification, ×390 (A to C) and ×580 (D).
Table 2.
Frozen Section Immunophenotyping in Four CD56+ and Nine CD56− Intestinal Lymphomas
| Case | βF1 | TCRδ-1 δ-TCS1 | CD3 | CD7 | CD2 | CD4 | CD8 | CD5 | CD56 | CD57 | CD103 | Perforin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD56+ cases | ||||||||||||
| 2 | − | − | − | + | + | − | + | − | + | − | − | + |
| 3 | − | − | + | + | + | − | + | − | + | − | + | − |
| 4 | + | − | + | +/− | i+ | − | − | + | + | − | − | 15% |
| 9 | −p | ND | + | ND | ND | − | −p | − | +p | − | − | ND |
| CD56− cases | ||||||||||||
| 16 | − | − | + | + | + | − | − | − | − | − | + | + |
| 17 | − | − | + | + | − | − | − | − | − | − | + | + |
| 23 | + | − | + | + | − | − | + | − | − | − | + | − |
| 26 | + | − | + | + | − | − | + | − | − | − | + | − |
| 29 | − | − | + | + | + | − | − | − | − | − | − | + |
| 42 | + | − | + | + | + | + | − | + | − | − | − | − |
| 44 | + | − | + | + | − | − | − | − | −p | −p | + | ND |
| 45 | − | − | + | + | − | − | − | − | − | − | − | ND |
| 46 | − | − | + | − | − | − | − | − | −p | −p | − | ND |
i, individual cells positive; p, reactivity on paraffin section if not done on frozen section; ND, not done.
Figure 2.
TCR γ-chain gene rearrangement of amplified genomic DNA extracted from paraffin sections of 15 CD56+ intestinal lymphomas. Numbers correspond to cases 1 to 15. One or two dominant bands indicating clonal mono- or biallelic rearrangements are present in all cases except in cases 2, 8, and 10. pCo, polyclonal control; +Co, positive control; −Co, negative control, no DNA added.
Histopathology of CD56+ Intestinal Lymphomas
Eleven of the fifteen CD56+ lymphomas showed strikingly uniform morphological features. At low-power magnification (not shown), these 11 lymphomas were characterized by the monotonous appearance of densely packed cells almost without any recognizable stroma compo-nents; at medium and high power, the monotonous character prevailed, because most of the rather monomorphic medium-sized cells (n = 8) or small to medium-sized cells (n = 3) contained only slightly irregular nuclei with small nucleoli and moderately wide, pale or sometimes clear cytoplasm (Figure 1D) ▶ . Conspicuous variation in cell size within a given tumor were not seen, and inflammatory cells, fibrosis, and necrotic changes were present in two cases only.
The remaining four CD56+ lymphomas were composed either of pleomorphic medium and large cells (n = 2), immunoblasts (n = 1), or pleomorphic small cells (n = 1).
Histopathology and Phenotype of 55 CD56− Intestinal Lymphomas
The majority of the 55 CD56− lymphomas were composed of pleomorphic medium and large cells or had a morphology most consistent with anaplastic large-cell lymphoma (ALCL). These tumors were frequently associated with fibrosis and admixed inflammatory cells. Prominent lymphoma-associated eosinophilia, as described by Shepherd et al, 17 was noted in nine cases. Four lymphomas showed the morphology of the monomorphic medium cell type described above and were thus indistinguishable from their CD56+ counterpart.
The immunohistochemical findings on paraffin and frozen sections are shown in Tables 1 and 2 ▶ ▶ , respectively. Only 2 of the 55 lymphomas were negative for both TIA-1 and GB-4; 27 expressed CD30, and 11 stained for EMA.
Detection of EBER Transcripts in CD56+ and CD56− Lymphomas
Six of the seventy cases showed nuclear EBER reactivity. However, a broad range of reactivity from <5% to >80% was noticed. In both immunophenotypic groups, one case each exhibited EBER positivity in >80% of the tumor cells throughout the lymphomatous infiltrate. Clustered EBER+ tumor cells were detected in three other cases; in a CD56− lymphoma, 50% of the tumor population was EBER+, and 10% to 15% reactivity was detected each in a CD56+ and a CD56− lymphoma. The remaining case of a CD56+ lymphoma contained <5% scattered small EBER+ cells, which did not allow distinction between tumor cells or reactive lymphocytes.
Distinguishing Features of CD56+ versus CD56− Intestinal Lymphomas
Distinguishing features of the two immunophenotypic groups are summarized and compared in Table 3 ▶ . In contrast to the CD56− cases, the CD56+ lymphomas were significantly more often positive for CD8 (80% versus 19%; P < 0.001) but constantly negative for CD30 and EMA. Morphologically, the CD56+ lymphomas were significantly more often of monomorphic small to medium-sized histology (73% versus 7%; P < 0.001), but significantly less often associated with histologically defined enteropathy (53% versus 85%; P = 0.03).
Table 3.
Comparison of Histological and Clinical Findings between CD56+ and CD56− Intestinal Lymphomas
| CD56+ | CD56− | P value* | |
|---|---|---|---|
| Histological findings | |||
| CD8+ | 12/15 (80) | 10/54 (19) | <0.001 |
| Monomorphic small to medium-sized histology (%) | 11/15 (73) | 4/55 (7) | <0.001 |
| Enteropathy (%) | 8/15 (53) | 44/52 (85) | 0.03 |
| Clinical findings | |||
| Median age (range) | 64 (42–82) | 61 (22–82) | |
| Sex (male/female) | 7/8 | 29/26 | |
| Preceding diagnosis of celiac disease (%) | 0/15 (0) | 16/50 (32) | 0.014 |
| Free intestinal perforation (%) | 10/15 (67) | 13/33 (39) | 0.12 |
| Histologically documented site(s) of lymphoma† (%) | |||
| Jejunum | 8 (53) | 30 (55) | |
| Ileum | 4 (27) | 9 (16) | 0.455 |
| Jejunum and ileum | 3 (20) | 11 (20) | |
| Small intestine‡ | 0 | 5 (9) | |
| Total | 15§ | 55¶ | |
| Stage of disease (%) | |||
| I–II | 11/15 (73) | 25/33 (76) | |
| III–IV | 4/15 (27) | 8/33 (24) | |
| Overall median survival in months (n) | 3 (15) | 3 (33) | 0.8789∥ |
* Fisher’s exact test (two-tailed).
† At initial diagnosis.
‡ Not otherwise specified.
§ One case with simultaneous gastric involvement.
¶ Eight cases with simultaneous involvement of stomach (n = 2) or colon (n = 6).
∥ Log-rank.
Comparison of Clinical Findings between Patients with CD56+ and CD56− Lymphomas
Clinical characteristics of the CD56+ and CD56− patients are summarized and compared in Table 3 ▶ . Age and sex distributions were almost identical in both groups. Four of the fifteen patients with CD56+ lymphoma had diarrhea for 1 week, 1 month, 14 months, and 15 months, respectively. None of the patients was known to have CD, although specific clinical and serological testing has been done only in the two patients with long-lasting diarrhea. In contrast, 16 of 50 patients with CD56− lymphoma had histories of CD ranging from 1 to 25 years (median, 6 years). This difference was statistically significant (0% versus 32%; P = 0.014). The CD56+ group had a higher incidence of free intestinal perforation at initial presentation. In both groups, the jejunum (either alone or in combination with ileal involvement) was the most common site of histologically documented lymphoma involvement, but CD56+ lymphomas were more often confined to the ileum. The two immunophenotypic groups were similar with regard to stage at initial presentation, having both stage I and II in ∼75% of the cases. Seventy-three per cent of patients with CD56+ lymphoma and 70% of patients with CD56− lymphoma died within 6 months. The majority of them did not receive chemotherapy or did not finish the complete course. Two patients with CD56+ lymphoma are alive, one patient with disease at 7 months and another disease-free at 85 months. One patient died of an unrelated cause at 86 months. Among 33 patients with CD56− lymphoma, 5 patients are alive, 1 with disease at 8 months and 4 who had all achieved complete remission after multiagent chemotherapy at 51, 56, 66, and 96 months, respectively. Overall median survival was 3 months for both groups.
Discussion
CD56 is a 200- to 220-kd glycoprotein expressed predominantly on human NK cells and a minor subset of T cells mediating major histocompatibility complex-nonrestricted cytotoxicity. 18 Lymphomas expressing CD56 are rare aggressive malignancies sharing the propensity for extranodal disease mostly involving the nasal or nasopharyngeal region, skin and subcutis, testis, and gastrointestinal tract. 10,11,19,20 Nasal/nasopharyngeal lymphoma, which may be considered the prototype of CD56+ lymphoma, is characterized by strong association with Epstein-Barr virus (EBV) and phenotypically by CD2+CD3/surface−CD3ε/cyt+CD56+, frequent absence of other T-lineage markers, and lack of clonal TCR gene rearrangement in most cases. 21 These tumors are currently referred to as nasal NK/T-cell lymphomas, 22 but there is growing evidence that they represent true NK-cell lymphomas. 23-26 The term nasal-type NK/T-cell lymphoma recognizes that there are lymphomas arising in a variety of extranodal sites that appear to be identical to nasal NK/T-cell lymphomas. 22
NK-like T cells are surface CD3+, express NK-cell antigens, such as CD56, and rearrange their TCR, which distinguishes them from true NK cells. 18,27 The extremely rare NK-like T-cell-derived lymphomas include the clinicopathological entity of hepatosplenic γδ T-cell lymphoma 28 and may, furthermore, arise at a variety of other extranodal sites among which the intestine could play an important role. 10,20,29,30
Normal human jejunal IELs are mainly TCRαβ+CD3+CD8+CD5low, and in situ analyses have shown lack of proteins carrying out cell-mediated cytolysis, such as granzyme B, perforin, and Fas ligand, indicating that IELs are resting cytotoxic T cells. 31,32 Upon activation, however, expression of granzyme B and Fas ligand is rapidly up-regulated. 32-34 Similar to the human liver, 35 jejunal IELs are also enriched for CD56+ T cells, constituting ∼15% of freshly isolated TCR-αβ+ IELs, which could be the cells of origin for CD56+ ITLs. 13 To test this hypothesis, seventy ITLs were studied for CD56 expression, and 15 CD56+ cases were identified. The common antigen profile in the majority of these lymphomas was βF1±CD3ε/cyt+CD8+CD4−CD5−CD56+, which is most consistent with NK-like T cells, and clonal TCR-γ gene rearrangement provided further evidence for T-cell derivation. Based on previous studies suggesting that ITLs arise from αβ-bearing T cells 2,4,8,36 and on the fact that only two well documented intestinal γδ T-cell lymphomas (both CD56−) have been reported, 37 it is conceivable that the vast majority of CD56+ ITLs are also of αβ T-cell origin. Nevertheless, a considerable proportion of putative αβ T-cell-derived ITLs in this and other reports did not stain for βF1, probably due to the insensitivity of the antibody. Alternatively, lack of βF1 reactivity could be a result of incomplete or nonproductive rearrangement of the β-chain, 4 or the tumor cells have indeed down-regulated their surface TCR, which has been shown in clonally expanded T-cell populations upon stimulation. 38,39
Three CD56+ lymphomas lacking detectable clonal TCR-γ gene rearrangements were probably true NK-cell lymphomas (cases 2 and 8) or of undetermined lineage (case 10), indicating that, in contrast to the sinonasal region, primary small-intestinal NK-cell lymphomas are exceptionally rare. 11,19,40 This finding is not unexpected as in the normal small intestine NK cells are virtually absent intraepithelially and rarely present in the lamina propria. 13,41
Expression of the activation-dependent cytotoxic molecules granzyme B and perforin, as well as reactivity for TIA-1, a granule-associated protein expressed by non-activated and activated NK cells and cytotoxic T cells, was demonstrated in this and previous studies, suggesting that ITLs are derived from activated cytotoxic T cells. 42-45
Recent studies on the presence of EBV in ITLs using sensitive in situ hybridization techniques have found a very low frequency of EBV+ European cases in contrast to a high incidence in cases of Mexican origin. 46-48 As an etiological role of EBV could be suggested only in those cases in which most if not all tumor cells are EBER+, 49 only 2 of our 70 cases (one CD56+) are clearly EBV associated. These data indicate that the virus is not implicated in the pathogenesis of European ITLs.
Eleven of the fifteen CD56+ lymphomas showed a strikingly similar histology characterized by densely packed monomorphic small to medium-sized cells, indicating a strong correlation between phenotype and morphology. Interestingly, the two lymphomas that could be of NK-cell origin (cases 2 and 8) also shared this histology, suggesting that lineage derivation might be of minor importance in this group of lymphomas. However, CD56 expression did not always predict morphology and vice versa, as a few CD56+ lymphomas showed other histologies and four monomorphic medium-sized cell lymphomas were unreactive to the CD56 antibody.
Some other features distinguishing CD56+ from CD56− ITLs were observed. The significantly less frequent expression of CD8 among the latter, which were composed predominantly of pleomorphic medium and large cells or had a morphology most consistent with ALCL, could be due to antigen loss or, alternatively, indicate that the majority of the CD56− ITLs are derived from the small subset of CD8−CD4−TCR-αβ T cells present in normal jejunum. 13 Histological evidence of enteropathy and a preceding diagnosis of CD were significantly less frequent in CD56+ lymphomas as compared with CD56− tumors. However, these findings should be interpreted with caution because CD-oriented clinical work-up was insufficient. Moreover, it is conceivable that in an unknown proportion of these putative non-EATCLs enteropathic changes have been missed because of their patchy distribution or have not been present at all, as recently described in patients with so-called latent (potential) CD. 50,51 Finally, and most important, no HLA data were available that could clearly demonstrate whether or not these cases were associated with the CD-HLA genotype. 52
In conclusion, the majority of CD56+ intestinal lymphomas are morphologically and phenotypically distinct T-cell lymphomas most likely derived from activated cytotoxic CD56+CD8+ IELs. There is, however, some overlap between CD56+ and CD56− ITLs in terms of presumptive cellular origin from the IEL compartment, histological appearance, clinical presentation, and outcome, suggesting that CD56+ intestinal lymphomas belong to the clinicopathological entity of ITL. The consistent expression of cytotoxic-granule-associated proteins introduces ITL (both CD56+ and CD56−) into the growing family of usually aggressive extranodal lymphomas of cytotoxic T-cell and NK-cell derivation. In contrast to putative NK-cell lymphoma of the sinonasal region, intestinal NK-cell lymphoma seems to be very rare, reflecting one of the unique properties of the intestinal mucosa-associated lymphoid tissue, which is almost devoid of NK cells.
Footnotes
Address reprint requests to Dr. Andreas Chott, General Hospital Vienna, Department of Clinical Pathology, Währinger Gürtel 18–20, A-1090 Vienna, Austria. E-mail: andreas.(chott@akh-wien.ac.at.
References
- 1.Isaacson PG, Wright DH: Intestinal lymphoma associated with malabsorption. Lancet 1978, i:67-70 [DOI] [PubMed] [Google Scholar]
- 2.Isaacson PG, Spencer J, Connolly CE, Pollock DJ, Stein H, O’Connor NTJ, Bevan DH, Kirkham N, Wainscoat JS, Mason DY: Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet 1985, ii:688-691 [DOI] [PubMed] [Google Scholar]
- 3.Salter DM, Krajewski AS, Dewar AE: Immunophenotype analysis of malignant histiocytosis of the intestine. J Clin Pathol 1986, 39:8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray A, Cuevas EC, Jones DB, Wright DH: Study of the immunohistochemistry and T cell clonality of enteropathy-associated T cell lymphoma. Am J Pathol 1995, 146:509-519 [PMC free article] [PubMed] [Google Scholar]
- 5.O’Farrelly C, Feighery C, O’Brian DS, Stevens F, Connolly CE, McCarthy C, Weir DG: Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br Med J 1986, 293:908-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer J, Cerf-Bensussan N, Jarry A, Brousse N, Guy-Grand D, Krajewsky AS, Isaacson PG: Enteropathy associated T-cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol 1988, 132:1-5 [PMC free article] [PubMed] [Google Scholar]
- 7.Stein H, Dienemann D, Sperling M, Zeitz M, Riecken EO: Identification of a T cell lymphoma category derived from intestinal-mucosa-associated T cells. Lancet 1988, ii:1053-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chott A, Dragosics B, Radaszkiewicz T: Peripheral T-cell lymphomas of the intestine. Am J Pathol 1992, 141:1361-1371 [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffe ES: Classification of natural killer (NK) cell and NK-like T-cell malignancies. Blood 1996, 87:1207-1210 [PubMed] [Google Scholar]
- 10.Macon WR, Williams ME, Greer JP, Hammer RD, Glick AD, Collins RD, Cousar JB: Natural killer-like T-cell lymphomas: aggressive lymphomas of T-large granular lymphocytes. Blood 1996, 87:1474-1483 [PubMed] [Google Scholar]
- 11.Chan KC, Sin VC, Wong KF, Ng CS, Tsang YW, Chan CH, Cheung MMC, Lau WH: Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood 1997, 89:4501-4513 [PubMed] [Google Scholar]
- 12.Marsh MN, Crowe PT: Morphology of the mucosal lesion in gluten sensitivity. Baillière’s Clin Gastroenterol 1995, 9:273-293 [DOI] [PubMed] [Google Scholar]
- 13.Lundquist C, Baranov V, Hammarström S, Athlin L, Hammarström M-L: Intra-epithelial lymphocytes: evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol 1995, 7:1473-1487 [DOI] [PubMed] [Google Scholar]
- 14.Frank SF, Svoboda-Newman SM, Hsi ED: Comparison of methods for extracting DNA from formalin-fixed paraffin sections for nonisotopic PCR. Diagn Mol Pathol 1996, 5:220-224 [DOI] [PubMed] [Google Scholar]
- 15.Födinger M, Buchmayer H, Schwarzinger I, Simonitsch I, Winkler K, Jäger U, Knobler R, Mannhalter C: Multiplex PCR for rapid detection of T-cell receptor-γ chain rearrangements in patients with lymphoproliferative diseases. Br J Haematol 1996, 94:136-139 [DOI] [PubMed] [Google Scholar]
- 16.Musshoff K: Klinische Stadieneinteilung der nicht-Hodgkin Lymphome. Strahlentherapie 1977, 153:218-221 [PubMed] [Google Scholar]
- 17.Shepherd NA, Blackshaw AJ, Hall PA, Bostad L, Coates PJ, Lowe DG, Lewison DA, Morson BC, Stansfeld AG: Malignant lymphoma with eosinophilia of the gastrointestinal tract. Histopathology 1987, 11:115-130 [DOI] [PubMed] [Google Scholar]
- 18.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH: The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 1986, 136:4480-4486 [PubMed] [Google Scholar]
- 19.Wong KF, Chan JKC, Ng CS, Lee KC, Tsang WYW, Cheung MMC: CD56 (NKH-1)-positive hematolymphoid malignancies: an aggressive neoplasm featuring frequent cutaneous/mucosal involvement, cytoplasmic azurophilic granules, and angiocentricity. Hum Pathol 1992, 23:798-804 [DOI] [PubMed] [Google Scholar]
- 20.Kern WF, Spier CM, Hannemann EH, Miller TP, Matzner M, Grogan TM: Neural cell adhesion molecule-positive peripheral T-cell lymphomas: a rare variant with a propensity for unusual sites of involvement. Blood 1992, 79:2432-2437 [PubMed] [Google Scholar]
- 21.Jaffe ES, Chan JKC, Su I-J, Frizzera G, Mori S, Feller AC, Ho FCS: Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas: definitions, differential diagnosis, and epidemiology. Am J Surg Pathol 1996, 20:103-111 [DOI] [PubMed] [Google Scholar]
- 22.Jaffe ES: Classification of natural killer (NK) cell and NK-like T-cell malignancies. Blood 1996, 87:1207-1210 [PubMed] [Google Scholar]
- 23.Weiss LM, Picker LJ, Grogan TM, Warnke RA, Sklar J: Absence of clonal β and γ T-cell receptor gene rearrangements in a subset of peripheral T-cell lymphomas. Am J Pathol 1988, 130:436-442 [PMC free article] [PubMed] [Google Scholar]
- 24.Suzumiya J, Takeshita M, Kimura M, Kikuchi M, Uchida T, Hisano S, Eura Y, Kozuro M, Nomura Y, Tomita K, Komiyama S, Okumura M: Expression of adult and fetal natural killer cell markers in sinonasal lymphomas. Blood 1994, 83:2255-2260 [PubMed] [Google Scholar]
- 25.Tao Q, Ho FCS, Loke SL, Srivastava G: Epstein-Barr virus is localized in the tumour cells of nasal lymphomas of NK, T or B cell type. Int J Cancer 1995, 60:315-320 [DOI] [PubMed] [Google Scholar]
- 26.Emile J-F, Boulland M-L, Haioun C, Kanavaros P, Petrella T, Delfau-Larue M-L, Bensussan A, Farcet J-P, Gaulard P: CD5− CD56+ T-cell receptor silent peripheral T-cell lymphomas are natural killer cell lymphomas. Blood 1996, 87:1466-1473 [PubMed] [Google Scholar]
- 27.Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA: Comparative studies of CD3− and CD3+CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol 1991, 136:486. [DOI] [PubMed] [Google Scholar]
- 28.Cooke CB, Krenacs L, Stetler-Stevenson M, Greiner TC, Raffeld M, Kingma DW, Abruzzo L, Frantz C, Kaviani M, Jaffe ES: Hepatosplenic T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic γδ T-cell origin. Blood 1996, 88:4265-4274 [PubMed] [Google Scholar]
- 29.Wasik MA, Sackstein R, Novick D, Butmarc JR, Zhang Q, Vonderheid EC, Kadin ME: Cutaneous CD56+ large T-cell lymphoma associated with high serum concentration of IL-2. Hum Pathol 1996, 27:738-744 [DOI] [PubMed] [Google Scholar]
- 30.Weiss RL, Lazarus KH, Macon WR, Gulley ML, Kjeldsberg CR: Natural killer-like T-cell lymphoma in the small intestine of a child without evidence of enteropathy. Am J Surg Pathol 1997, 21:964-969 [DOI] [PubMed] [Google Scholar]
- 31.Trejdosiewicz LK: Intestinal intraepithelial lymphocytes and lymphoepithelial interactions in the human gastrointestinal mucosa. Immunol Lett 1992, 32:13-20 [DOI] [PubMed] [Google Scholar]
- 32.Chott A, Gerdes D, Spooner A, Mosberger I, Kummer JA, Ebert CE, Blumberg RS, Balk SP: Intraepithelial lymphocytes in normal human intestine do not express proteins associated with cytolytic function. Am J Pathol 1997, 151:435-442 [PMC free article] [PubMed] [Google Scholar]
- 33.Oberhuber G, Vogelsang H, Stolte M, Muthenthaler S, Kummer AJ, Radaszkiewicz T: Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am J Pathol 1996, 148:1351-1357 [PMC free article] [PubMed] [Google Scholar]
- 34.Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P: CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 1997, 113:160-167 [DOI] [PubMed] [Google Scholar]
- 35.Winnock M, Garcia-Barcina M, Lukomska B, Huet S, Saric J, Balabaud C, Bioulac-Sage P: Human liver-associated lymphocytes: an overview. J Gastroenterol Hepatol 1995, 10(Suppl 1):43-46 [DOI] [PubMed] [Google Scholar]
- 36.Schmitt-Gräff A, Hummel M, Zemlin M, Schneider T, Ullrich R, Heise W, Zeitz M, Riecken E-O, Stein H: Intestinal T-cell lymphoma: a reassessment of cytomorphological and phenotypic features in relation to patterns of small bowel remodelling. Virchows Arch 1996, 429:27-36 [DOI] [PubMed] [Google Scholar]
- 37.Arnulf B, Copie-Bergmann C, Delfau-Larue M-H, Lavergne-Slove A, Bosq J, Wechsler J, Wassef M, Matuchansky C, Epardeau B, Stern M, Bagot M, Reyes F, Gaulard P: Nonhepatosplenic γδ T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood 1998, 91:1723-1731 [PubMed] [Google Scholar]
- 38.Ferber I, Schönrich G, Schenkel J, Mellor AL, Hämmerling GJ, Arnold B: Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science 1994, 263:674-676 [DOI] [PubMed] [Google Scholar]
- 39.Niedergang F, Hemar A, Hewitt CRA, Owen MJ, Dautry-Varsat A, Alcover A: The Staphylococcus aureus enterotoxin B superantigen induces specific T cell receptor down-regulation by increasing its internalization. J Biol Chem 1995, 270:12839-12845 [DOI] [PubMed] [Google Scholar]
- 40.Martin AR, Chan WC, Perry DA, Greiner TC, Weisenburger DD: Aggressive natural killer cell lymphoma of the small intestine. Mod Pathol 1995, 8:467-472 [PubMed] [Google Scholar]
- 41.Van Tool EAF, Verspaget HWV, Peña AS, Kraemer CVE, Lamers CBHW: The CD56 adhesion molecule is the major determinant for detecting non-major histocompatibility complex-restricted cytotoxic mononuclear cells from the intestinal lamina propria. Eur J Immunol 1992, 22:23-29 [DOI] [PubMed] [Google Scholar]
- 42.De Bruin PC, Kummer JA, Van der Valk P, Van Heerde P, Kluin PM, Willemze R, Ossenkoppele GJ, Radaszkiewicz T, Meijer CJLM: Granzyme B-expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood 1994, 84:3785-3791 [PubMed] [Google Scholar]
- 43.Daum S, Foss H-D, Anagnostopoulos I, Dederke B, Demel G, Araujo I, Riecken E-O, Stein H, : German Study Group on Intestinal Non-Hodgkin Lymphoma: Expression of cytotoxic molecules in intestinal T-cell lymphomas. J Pathol 1997, 182:311-317 [DOI] [PubMed] [Google Scholar]
- 44.De Bruin PC, Connolly CE, Oudejans JJ, Kummer JA, Jansen W, McCarthy CF, Meijer CJLM: Enteropathy-associated T-cell lymphomas have a cytotoxic T-cell phenotype. Histopathology 1997, 31:313-317 [DOI] [PubMed] [Google Scholar]
- 45.Felgar RE, Macon WR, Kinney MC, Roberts S, Pasha T, Salhany KE: TIA-1 expression in lymphoid neoplasms: identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol 1997, 150:1893-1900 [PMC free article] [PubMed] [Google Scholar]
- 46.Ilyas M, Niedobitek G, Agathanggelou A, Barry RE, Read AE, Tierney R, Young LS, Rooney N: Non-Hodgkin’s lymphoma, coeliac disease, and Epstein-Barr virus: a study of 13 cases of enteropathy-associated T- and B-cell lymphoma. J Pathol 1995, 177:115-122 [DOI] [PubMed] [Google Scholar]
- 47.Walsh SV, Egan LJ, Connolly CE, Stevens FM, Egan EL, McCarthy CF: Enteropathy-associated T-cell lymphoma in the West of Ireland: low-frequency of Epstein-Barr virus in these tumors. Mod Pathol 1995, 8:753-757 [PubMed] [Google Scholar]
- 48.Quintanilla-Martinez L, Lome-Maldonado C, Ott G, Gschwendtner A, Gredler E, Reyes E, Angeles-Angeles A, Fend F: Primary non-Hodgkin’s lymphoma of the intestine: high prevalence of Epstein-Barr virus in Mexican lymphomas as compared with European cases. Blood 1997, 89:644-651 [PubMed] [Google Scholar]
- 49.Kanavaros P, Briere J, Emile JF, Gaulard P: Epstein-Barr virus in T and natural killer (NK) cell non-Hodgkin’s lymphomas. Leukemia 1996, 10:s84-87 [PubMed] [Google Scholar]
- 50.Arranz E, Ferguson A: Intestinal antibody pattern of celiac disease: occurrence in patients with normal jejunal biopsy histology. Gastroenterology 1993, 104:1263-1272 [DOI] [PubMed] [Google Scholar]
- 51.Picarelli A, Maiuri L, Mazzilli MC, Coletta S, Ferrante P, Di Giovambattista F, Greco M, Torsoli A, Auricchio S: Gluten-sensitive disease with mild enteropathy. Gastroenterology 1996, 111:608-616 [DOI] [PubMed] [Google Scholar]
- 52.Howell WM, Leung ST, Jones DB, Nakshabendi I, Hall MA, Lanchbury JS, Ciclitira PJ, Wright DH: HLA-DRB, -DQA, and -DQB polymorphism in celiac disease and enteropathy-associated T-cell lymphoma: common features and additional risk factors for malignancy. Hum Immunol 1995, 43:29-37 [DOI] [PubMed] [Google Scholar]