Abstract
Expression of the β3 subunit of the αvβ3 vitronectin receptor on melanoma cells is associated with tumor thickness and the ability to invade and metastasize. To address the role of αvβ3 in the complex process of progression from the nontumorigenic radial to the tumorigenic vertical growth phase of primary melanoma, we examined the biological consequences of overexpressing αvβ3 in early-stage melanoma cells using an adenoviral vector for gene transfer. Overexpression of functional αvβ3 in radial growth phase primary melanoma cells 1) promotes both anchorage-dependent and -independent growth, 2) initiates invasive growth from the epidermis into the dermis in three-dimensional skin reconstructs, 3) prevents apoptosis of invading cells, and 4) increases tumor growth in vivo. Thus, αvβ3 serves diverse biological functions during the progression from the nontumorigenic radial growth phase to the tumorigenic and invasive vertical growth phase primary melanoma.
The easy accessibility and long-term clinical and histopathological observation of cutaneous melanoma has led to the definition of five major steps of tumor progression in the human melanocytic system. The lesions representing each step are common acquired nevus, dysplastic nevus, radial growth phase (RGP) primary melanoma, vertical growth phase (VGP) primary melanoma, and metastatic melanoma. 1,2 The common acquired nevus is composed of nests of mature melanocytes. Increasing levels of cytological and architectural atypia and aberrant cell growth are observed in dysplastic nevi. The RGP primary melanoma is the first recognizable malignant step, but the malignant cells grow only within or in close proximity to the epidermis and they do not have competence for metastasis. 2 Eventually, cells acquire the ability to invade deeply into the dermis (VGP), a stage that is associated with metastatic dissemination and dependent on tumor thickness, mitotic index, and degree of lymphocytic infiltration. 2,3 Thus, the conversion of primary melanomas from RGP to VGP is the most critical step in melanoma progression and ultimately in disease outcome.
The successful isolation and in vitro propagation of cells derived from different stages of progression has provided an excellent experimental model for studying tumor progression. 4-8 Cultured cells from RGP and VGP progression stages have biological properties that reflect the stage in vivo. Cells with RGP-like phenotype 1) require complex media containing several growth factors for continuous proliferation in culture due to their limited autoexpression of growth factors, 7 2) do not grow in soft agar or form very few colonies, 4,7 3) show a heterogeneous response to keratinocyte-mediated control of growth and regulation of cell surface receptor expression, 9 and 4) are nontumorigenic or grow very slowly in immunodeficient nude or SCID mice to a small tumor mass (<150 μg) over 3 to 4 months. 7 These results are consistent with analyses from patients indicating that RGP primary melanoma cells have a nonmalignant phenotype relatively similar to precursor cells. 3 However, RGP primary melanoma cells are clearly distinguished from normal melanocytes or nevus cells by prolonged survival in culture or, as we have demonstrated with seven cell lines, by indefinite growth. 7 Most, but not all, cell lines are also independent of basic fibroblast growth factor (bFGF) and the phorbol ester TPA for growth and survival. 7
In contrast to cells from RGP lesions, those with a VGP-like phenotype are readily adapted to growth in tissue culture 8 and need, at most, one growth factor (insulin-like growth factor (IGF)-1 or insulin) for proliferation. 10 VGP cells express a variety of growth factors for either autocrine or paracrine stimulation 11 and readily adapt to growth in growth-factor-free medium, leading to increased invasiveness through basement membranes in vitro and metastasis formation in vivo. 12 VGP cells also form colonies in soft agar, 4 do not respond to growth control by keratinocytes, 9 and are tumorigenic in immunodeficient mice, with continuous local growth until the host dies. 5,13
Integrins constitute a family of membrane glycoproteins that are responsible for cell-extracellular matrix and cell-cell adhesion. Accumulating evidence points to the role of integrins as signal transducers in a variety of cellular events, including migration, proliferation, survival, invasion, differentiation, and matrix remodeling. 14-18 Given their potential as diagnostic and prognostic markers and as therapeutic targets, much effort has focused on the differences in integrin profiles between normal and malignant melanocytes. 18-20 Among the most consistent observation is the up-regulation of the β3 subunit of the αvβ3 vitronectin receptor in VGP melanoma in situ. 21-24 In addition, expression of αvβ3 correlates with clinical recurrence and mortality. 25,26 Immunohistochemical studies using subunit-specific antibodies revealed that, in contrast to the selective expression of β3 subunit in advanced melanoma, the αv subunit is detected in all stages. 21,23,26,27 The αv subunit forms complexes with β1, β3, β5, and β6, whereas β3 in melanoma predominantly pairs with αv. Other experimental approaches, including comparison of cell variants with different levels of αvβ3 expression 23,28,29 and perturbation of αvβ3 function with antibodies or peptides 30-32 have further suggested the contribution of αvβ3 to melanoma growth, invasion, and metastasis. However, recent transfection studies aimed at dissecting the biological role of the αv and β3 subunits in melanoma progression have yielded conflicting results; transfection of αv cDNA into an αv-deficient melanoma variant restored tumorigenicity 33 and promoted cell growth and survival in three-dimensional collagen gel, 34 whereas ectopic expression of β3 in a β3-negative but highly metastatic human melanoma cell line inhibited invasion and experimental metastasis. 35
To further investigate the potential role of αvβ3 in melanoma progression, we overexpressed the β3 subunit in RGP-like primary melanoma cell lines using replication-deficient adenoviruses as a gene delivery vehicle. We find that functional expression of the αvβ3 integrin receptor potentiates the malignant phenotype in vitro and in vivo. In three-dimensional skin reconstructs where the physiological melieu is recreated in vitro, induced β3 expression triggers an invasive phenotype and prevents apoptosis. In vivo, β3 overexpression induces tumor growth.
Materials and Methods
Cell Culture
Human melanoma cell line WM1552C, which has an RGP-like phenotype, was isolated as described 3,28,29 from a superficial spreading melanoma lesion. SBcl2, a primary melanoma cell line with an RGP-like phenotype, was a gift from Dr. B. Giovanell (Stehlin Foundation for Cancer Research, St. Joseph Hospital, Houston, TX). WM1341D is a VGP-like cell line, 8 which constitutively expresses αvβ3. All cell lines were maintained in W489 medium consisting of 4 parts MCDB153 supplemented with 2 mmol/L CaCl2 and 1 part L-15, 5 μg/ml insulin, and 2% fetal bovine serum (FBS). Keratinocytes were isolated from foreskin and grown in serum-free keratinocyte growth medium (KGM) containing modified MCDB153 36 supplemented with bovine pituitary extract (BPE; 140 μg/ml), epidermal growth factor (EGF; 10 ng/ml), ethanolamine (0.1 mmol/L), hydrocortisone (5 × 10−7 mol/L), insulin (5 μg/ml), and O-phosphoryl ethanolamine (0.1 mmol/L). Primary human dermal fibroblasts were initiated as explant cultures from trypsin-treated and epidermis-stripped neonatal foreskin. These cells were passaged in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS. The trans-complementing 293 cells, a cell line immortalized and transformed by adenovirus E1a and E1b, respectively, were obtained from the Vector Core at the Institute for Human Gene Therapy, University of Pennsylvania, Philadelphia, PA, and maintained in DMEM with 10% FBS. All tissue culture reagents were purchased from Sigma Chemical Co. (St. Louis, MO) except for EGF (Collaborative Biomedical Products, Bedford, MA) and L-15 and DMEM (Gibco-BRL, Gaithersburg, MD).
Construction of β3/Ad5
Adenoviral vector β3/Ad5 was constructed essentially as described. 37 Briefly, a 4.0-kb EcoRI fragment containing the entire coding sequence of human integrin β3 subunit in pBluescript was subcloned into EcoRI-cut pAd.CMV-Link.1 (obtained from the Vector Core, Institute for Human Gene Therapy). The resulting shuttle vector was linearized with NheI and co-transfected with ClaI-cut, E1–E3-deleted dl7001 human adenoviral DNA into 293 cells by standard calcium phosphate precipitation. Two days after transfection, cells were overlaid with 0.8% agar in MEM (Gibco-BRL) and fed every 3 to 4 days. Individual plaques were picked and screened for β3 expression by immunoprecipitation with monoclonal antibody (MAb) SSA6 (kindly provided by Dr. J. Hoxie, University of Pennsylvania). 38 Positive clones were subjected to three rounds of plaque purification to eliminate contamination with wild-type virus. Plaque-purified viruses propagated in 293 cells (β3/Ad5) were purified by ultracentrifugation in a cesium chloride gradient as described. 37 Viral titer was evaluated by absorbance at 260 nm, and the activity was assessed by plaque formation in permissive 293 cells.
Radiolabeling and Immunoprecipitation
Cells were infected at a multiplicity of infection of 10 and metabolically labeled by overnight incubation in methionine-free DMEM supplemented with 25 μCi/ml [35S]methionine (Amersham, Arlington Heights, IL). Cells were washed with PBS and extracted with non-ionic detergent buffer (10 mmol/L Tris/acetate, pH 8.0, 150 mmol/L NaCl, 0.5% Nonidet P-40, 0.5 mmol/L CaCl2) containing a protease inhibitor (2 mmol/L phenylmethylsulfonyl fluoride). Cell extracts were clarified by centrifugation at 14,000 × g for 20 minutes and precleared with protein-A-conjugated Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) for 30 minutes at 4°C. Precleared cell extracts were normalized according to radioactivity, and 100 μl was incubated with 1 μg of β3-specific SSA6 MAb for 1 hour at 4°C. Protein-A-conjugated Sepharose beads were then added to the immune complexes. The mixture was incubated for an additional hour at 4°C followed by washing five times with DOC wash (50 mmol/L Tris/HCl, pH 7.5, 150 mmol/L 1% Triton X-100, 5% deoxycholate, and 0.1% SDS). Antigens were released from the beads by boiling in Laemmli sample buffer. Samples were separated on 6% polyacrylamide gels under nonreduced conditions. Gels were dried and exposed to x-ray film.
Flow Cytometry
Infected cells were trypsinized, washed, and resuspended in serum-free DMEM with 10 μg/ml MAb SSA6. After 1 hour of incubation at 4°C with gentle rocking, cells were washed to remove unbound antibodies and stained with 10 μg/ml fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 minutes at 4°C. After washing, cells were resuspended in PBS and analyzed by fluorescence-activated cell sorting (FACS) using an Ortho Cytofluorograf 50H connected to a 2150 Data Handling System (Ortho Diagnostics, Westwood, MA).
Anchorage-Dependent Growth Assay
Cells from subconfluent cultures were trypsinized and seeded in triplicate 35-mm wells at 2 × 105 cells/well. The medium was changed twice a week. At different time points, cells were harvested and counted in a Coulter counter (Coulter Electronics, Luton, UK). All assays were performed in triplicate wells.
Soft Agar Growth
To prevent cell attachment, 1 ml of 0.5% Agar Nobel (Difco Laboratories, Detroit, MI) in WM489 medium supplemented with 50 μg/ml BPE, 3.5 ng/ml EGF, and 7% FBS was placed in six-well tissue culture plates and allowed to gel at room temperature. Subconfluent cultures were harvested by trypsinization, resuspended to 30,000 cells/ml in W489 medium supplemented with 5 ng/ml EGF and 70 μg/ml BPE, and mixed with agar to a final concentration of 6000 cells/well in 0.25% agar. Triplicate wells were prepared for each group of transduced and nontransduced cells.
Invasion and Cell Survival in Skin Reconstructs
Skin reconstructs were prepared as described 39-41 with modifications. Briefly, subconfluent dermal fibroblasts isolated from foreskins were harvested and resuspended to 1.5 × 105 cells/ml in DMEM with 10% FBS and 1 mg/ml neutralized rat tail collagen (Collaborative Biomedical Products). Three milliliters of the mixture was then seeded onto Transwell inserts (Corning Costar Corp., Cambridge, MA) placed in six-well tissue culture plates with 1 ml of precast acellular collagen and incubated at 37°C. After 6 days, fibroblasts had contracted collagen gels, creating a concave surface that served as a cradle for seeding epidermal cells. This portion represented the dermal reconstruct. The expelled medium was suctioned off, and the dermal reconstruct was equilibrated in epidermal growth medium (EGM) composed of 3 parts DMEM, 1 part Ham’s F-12, and 0.3% dialyzed newborn calf serum supplemented with 10 ng/ml EGF, 1.88 mmol/L CaCl2, 0.18 mmol/L adenine, 4 mmol/L glutamine, 53 nmol/L selenic acid, 0.1 mmol/L ethanolamine, 0.1 mmol/L O-phosphoryl ethanolamine, 5 μg/ml insulin, 5 μg/ml transferrin, 20 pmol/L tri-iodothyronine, 0.4 μg/ml hydrocortisone, 10 nmol/L progesterone, and 1.5 mmol/L HEPES for 1 hour at 37°C. The medium was discarded, and dermal reconstructs were dried at room temperature for 30 minutes. Melanoma cells were trypsinized and washed in Ca2+/Mg2+-free HEPES-buffered saline solution three times before mixing with keratinocytes at a 1:5 ratio in EGM to yield a cell concentration of 3 × 106/ml, and 50 μl of cell suspension was seeded onto the dry surface of dermal constructs. After 2 hours, cultures were submerged and re-fed every 2 to 3 days thereafter. Five days after seeding, medium was switched to maintenance medium (1:1 mixture of DMEM and Ham’s F12 supplemented with 1% newborn calf serum, 1.95 mmol/L CaCl2, 0.18 mmol/L adenine, 4 mmol/L glutamine, 53 nmol/L selenic acid, 0.1 mmol/L ethanolamine, 0.1 mmol/L O-phosphoryl ethanolamine, 5 μg/ml insulin, 5 μg/ml transferrin, 20 pmol/L triiodothyronine, 0.4 μg/ml hydrocortisone, and 1.5 mmol/L HEPES), and cultures were lifted to the air-liquid level to allow further epidermal stratification for another 10 days with regular feeding. Skin reconstructs were then harvested, fixed in 4% paraformaldehyde, and embedded in paraffin. The invasive capacity of melanoma cells was determined by morphological evaluation using hematoxylin and eosin staining. Cell survival after invasion into dermal reconstructs was assessed using an ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD).
Tumorigenicity
Melanoma cells were suspended at 3 × 108/ml in growth medium. Female SCID mice (five mice per group) were injected subcutaneously in the back with 100 μl of cell suspension. Tumor volume was determined as follows: (maximal dimensions × minimal dimensions)2/2.
Results
Functional Expression of αvβ3 Integrin by Melanoma Cells
Adenoviral vector β3/Ad5 induced cell surface expression of β3 within 48 hours of cell infection at a multiplicity of infection of 10 to 20, as evidenced by the increase in positively stained cells from 8.9% to 67.8% and 0.9% to 90.4% in WM1552C and SBcl2 cells, respectively, in FACS analysis using lacZ/Ad5-infected cells as controls (Figure 1A) ▶ . Little overexpression was found in WM1341D cells, which constitutively express αvβ3. Immunoprecipitation analysis using a β3-specific MAb and extracts of radiolabeled cells normalized according to radioactivity revealed bands with molecular masses corresponding to αv and β3 in all samples (Figure 1B) ▶ . Consistent with the FACS data, β3/Ad5 induced a marked increase in αvβ3 expression in WM1552C (lanes 3 and 4) and SBcl2 (lanes 1 and 2) cells as compared with their control virus-infected counterparts. Again, further up-regulation of β3 in αvβ3-expressing WM1341D cells by β3/Ad5 was limited (lanes 5 and 6). In addition, immunoblotting of β3 precipitates using an αv-specific MAb (a kind gift of Dr. S. L. Goodman, E. Merck, Darmstadt, Germany) confirmed a functional association between transduced β3 and endogenous αv in β3/Ad5-infected SBcl2 cells (data not shown).
Figure 1.
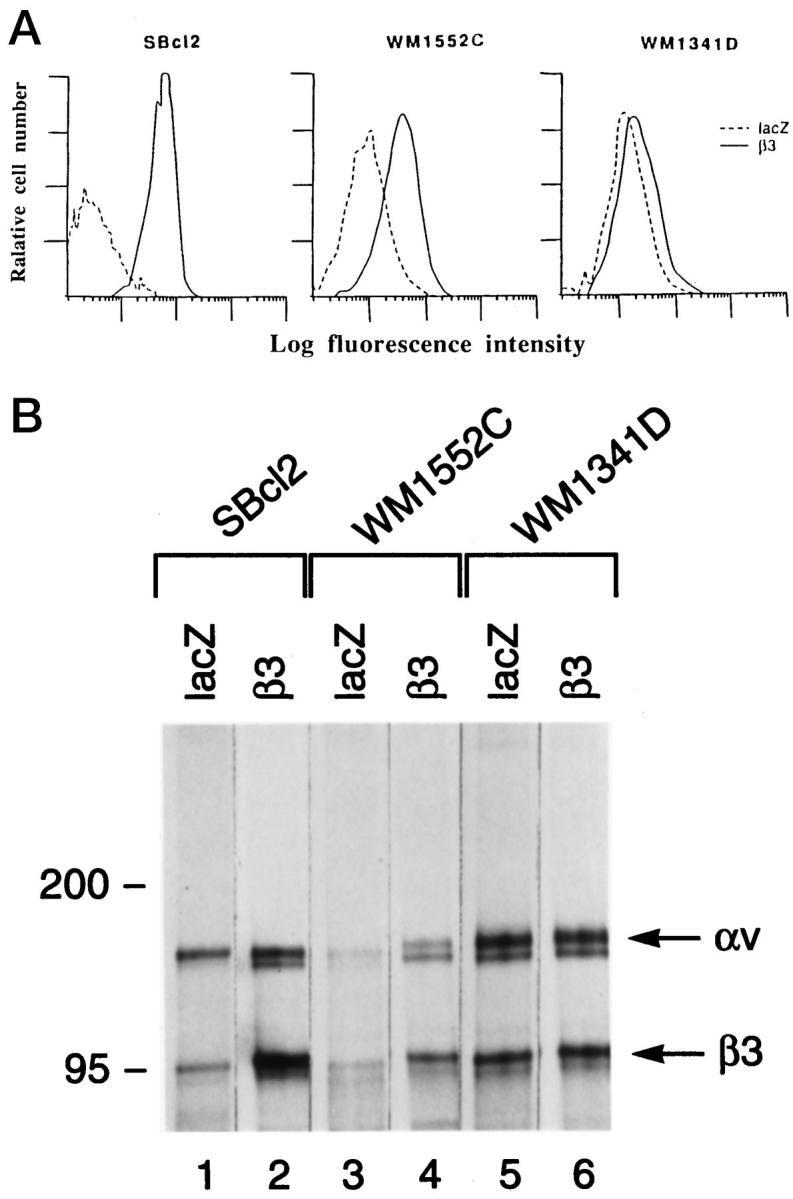
Induction of β3 expression by β3/Ad5. A: Cell surface expression of virally transduced β3 integrin subunit. Virus-infected melanoma cells were trypsinized, sequentially incubated with anti-β3 MAb (SSA6) and FITC-conjugated goat anti-mouse IgG, and analyzed by flow cytometry. The x axis indicates relative fluorescence intensity (log units); the y axis shows the relative cell number. - - - and ——, lacZ/Ad5- and β3/Ad5-infected cells, respectively. B: Complex formation of virus-induced β3 with endogenous αv. Cell lysates of infected melanoma cells were prepared after metabolic labeling, normalized for radioactivity, immunoprecipitated with β3-specific SSA6 MAb, and subjected to electrophoresis. Gels were fixed, dried, and exposed to x-ray film.
Induction of β3 Expression Promotes Melanoma Growth in Vitro
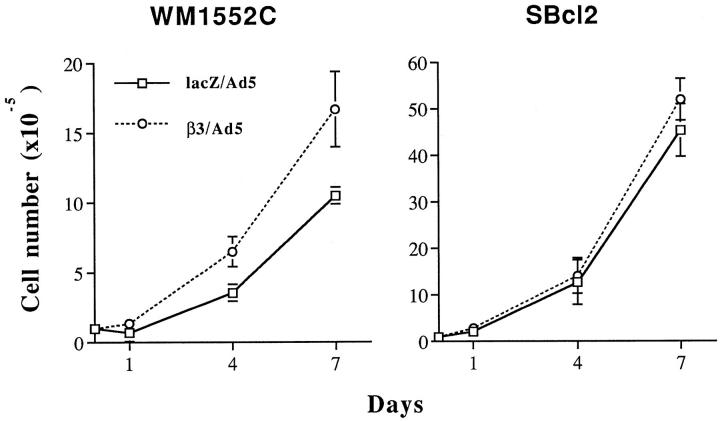
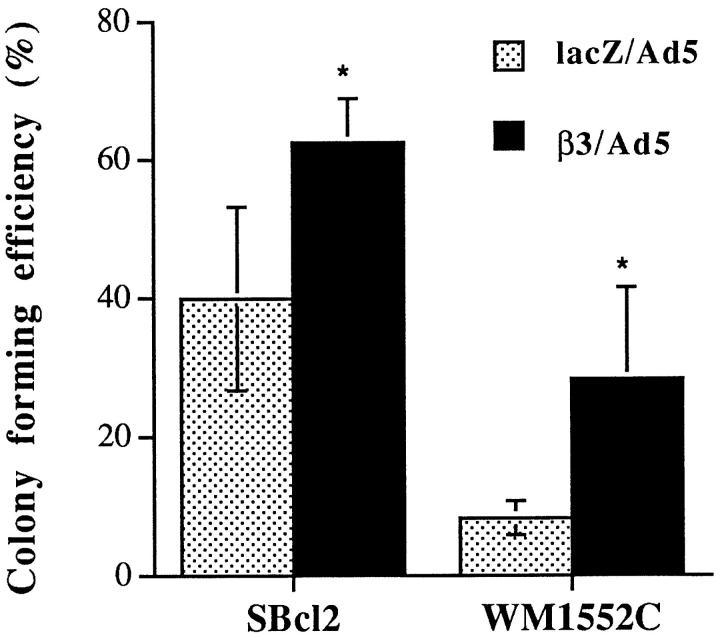
To investigate the role of the β3 integrin subunit in growth regulation of melanoma cells in vitro, cell proliferation in monolayer culture and in soft agar was examined. Growth of monolayer cultures was stimulated (twofold) in β3-transduced WM1552C cells but not in SBcl2 cells (Figure 2) ▶ , whereas in soft agar assays, colony-forming efficiencies were enhanced in both cell lines, with a 3.5- and 1.5-fold increase in WM1552C and SBcl2 cell growth, respectively (Figure 3) ▶ .
Figure 2.
In vitro growth of melanoma cells after β3 overexpression. Two days after viral infection, 2 × 105 cells were seeded into six-well tissue culture plates. Cell growth was monitored on days 1, 4, and 7 using a Coulter counter. Average cell number from triplicate wells was plotted for WM1552 and SBcl2 cells.
Figure 3.
Cell growth in soft agar of melanoma cells overexpressing β3. Melanoma cells were infected at a multiplicity of infection of 20 and, 2 days later, resuspended in 0.25% agar, seeded on an acellular layer, and fed regularly. After 3 to 4 weeks of culture, colony-forming efficiency was determined as the percentage of cells forming colonies containing four or more cells. Ten randomly chosen high-power fields were examined for each condition. *Statistical significance using Student’s t-tests; P values were 0.009 and 0.007 for SBcl2 and WM1552C cells, respectively.
Invasion and Survival in Three-Dimensional Skin Reconstructs
As integrin function is profoundly influenced by the extracellular milieu, 42-49 we examined melanoma invasion under physiological conditions by incorporating the transduced cells into three-dimensional skin reconstructs. Skin reconstructs consist of artificial skin rebuilt from isolated cell populations and composed of a stratified, terminally differentiated epidermal compartment of keratinocytes and melanocytes, a dermal compartment consisting of fibroblasts embedded in collagen gel, and a well established basement membrane deposited by skin cells. 41 β3/Ad5-infected SBcl2 cells invaded deep into the dermis and formed cell nests (Figure 4, A and C) ▶ , whereas lacZ/Ad5-infected cells spread only horizontally (Figure 4, B and D) ▶ . Moreover, lacZ/Ad5-infected cells showed the clear morphological signs of apoptosis, including nuclear condensation, membrane blebbing, and apoptotic bodies (Figure 4D) ▶ . These cells exhibited intense ApopTag staining, confirming their apoptotic cell death (Figure 4F) ▶ , whereas β3-expressing SBcl2 cells were completely negative (Figure 4E) ▶ .
Figure 4.
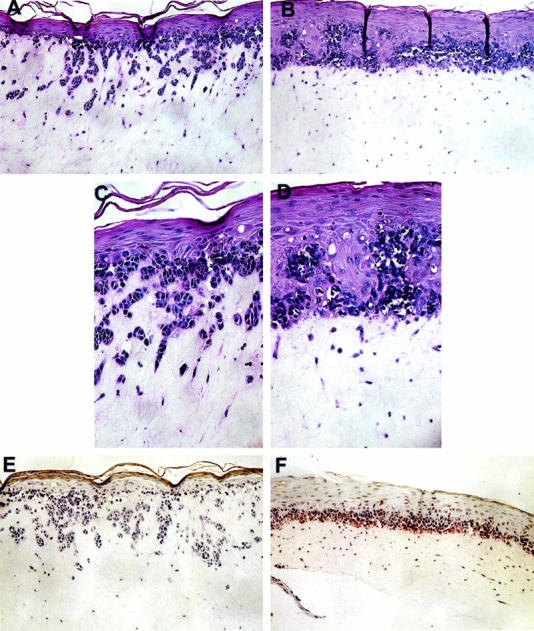
Effect of β3 overexpression on melanoma invasion and survival in three-dimensional skin reconstructs. Virus-infected SBcl2 melanoma cells were incorporated into the epidermis of skin reconstructs as described in Materials and Methods. Mature reconstructs were harvested, fixed, and embedded in paraffin. β3/Ad5-infected SBcl2 cells grew in an invasive pattern reminiscent of VGP primary melanoma (A and C), whereas lacZ/Ad5-infected cells spread horizontally, resembling RGP primary melanoma (B and D). Control virus-infected cells at the dermal/epidermal junction displayed apoptotic features, including nucleus condensation, membrane blebbing, and presence of apoptotic bodies. β3/Ad5-infected cells were completely negative for staining by the ApopTag in situ apoptosis detection kit (E), whereas lacZ/Ad5-infected cells stained positive (F). Magnification, ×100 (A, B, E, and F) and ×259 (C and D).
Tumorigenicity in Vivo of Early-Stage Melanoma Cells Expressing β3 Subunit
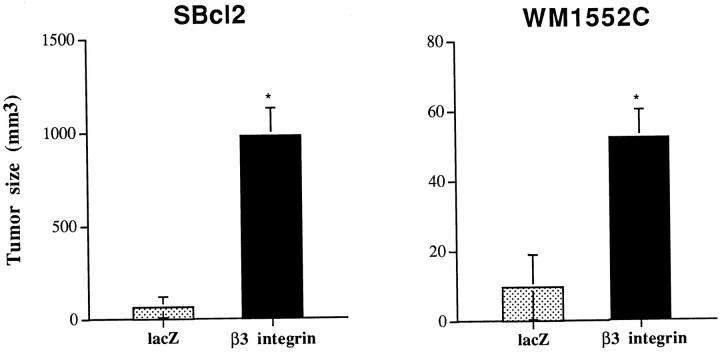
To study the biological consequences of β3 overexpression in early-stage, nontumorigenic melanoma in vivo, tumorigenicity of virus-transduced cells was evaluated in SCID mice injected subcutaneously with 3 × 10 7 β3/Ad5- or lacZ/Ad5-infected cells. The average size of tumors formed by β3-expressing cells at 7 days after injection was 5-fold (WM1552C) and 15-fold (SBcl2) larger than their lacZ/Ad5-infected counterparts (Figure 5) ▶ . However, due to the episomal nature of adenoviral vectors, all tumors began disappearing after day 10.
Figure 5.
Tumorigenicity of melanoma cells overexpressing β3 subunit. Ad5-infected cells were trypsinized and resuspended in growth medium. SCID mice were injected subcutaneously with 3 × 10 6 cells/mouse in a volume of 100 μl (five mice/group), and tumor size was determined at day 7. Student’s t-tests confirmed statistically significant differences between groups with P values <0.05 (SBcl2, P = 0.006; WM1552C, P = 0.001).
Discussion
Comparative analyses of integrin expression in different stages of melanoma have identified the onset of β3 integrin expression as one of the most specific markers of the transition from RGP to VGP primary and metastatic melanoma. 21-24 We find here that forced expression of the β3 subunit results in a functional complex with endogenous αv that potentiates the malignant phenotype reminiscent of the progression from RGP to VGP; ie, β3-expressing cells exhibit a growth advantage in monolayer cultures and soft agar, increased invasiveness and survival in skin reconstructs in vitro, and enhanced tumorigenicity in vivo.
Previous transfection studies showed that introduction of either subunit of the αvβ3 receptor into metastatic melanoma cell lines did not affect cell proliferation in vitro. 33,35 In contrast, we find that overexpression of β3 stimulates in vitro growth of WM1552C cells. This is not surprising as interplay between αvβ3 and growth factor receptors such as insulin and IGF receptors 50 as well as platelet-derived growth factor (PDGF)-β receptors 51 has been shown to control cell growth. However, the same growth-promoting effect was not seen in SBcl2 cells, which have a more rapid basal proliferation rate than that of WM1552C cells (Figure 2) ▶ . Thus, mechanisms other than αvβ3 appear to be involved in SBcl2 cell growth stimulation. Indeed, given the apparent redundancy of integrins, it is conceivable that functions of αvβ3 are replaced in part by α5β1, 46 αvβ1, αvβ5, or αvβ6, 52 which are all present in melanoma cells.
A hallmark of malignant transformation is anchorage-independent growth of the cells. By introducing the β3 integrin subunit, we demonstrated an increase in colony-forming efficiency of early-stage melanoma cells in soft agar. The mechanism(s) of this growth advantage after β3 transduction are not clear. In melanoma, cell-cell interactions occur through the adhesion receptor Mel-CAM, a member of the Ig gene superfamily, which binds to an unknown ligand also expressed by melanoma cells. 9,53 αvβ3 expressed by melanoma cells can bind to L1, another member of the Ig gene superfamily found on melanoma cells. 34 It is possible that such cell-cell interactions may provide signals for anchorage-independent survival and growth.
Progressive invasion into the dermis is one of the most important characteristics of VGP melanoma cells. This process requires disassociation of melanoma cells from neighboring keratinocytes and attachment to and proteolytic degradation of basement membrane components, followed by invasion and proliferation in the dermis. In our skin reconstruct model, which, unlike traditional invasion assays, accounts not only for tumor-cell-derived mechanisms but also for microenvironmental factors from stromal cells, control virus-infected SBcl2 cells grew in a pattern resembling RGP lesions, whereas β3-expressing cells showed a VGP growth pattern. The latter cells invaded and proliferated deep in the dermis without signs of apoptotic change whereas control cells remained in the epidermis, dying by apoptosis in the dermis. Coordinate expression and activation of αvβ3 with that of matrix metalloproteinases and urokinase-type plasminogen activator receptor 46,47 may play a crucial role in proteolysis of the extracellular matrix during invasion. Indeed, activated matrix metalloproteinase (MMP)-2 binds to cell surface αvβ3, thereby localizing its enzymatic activity to the leading edge of tumor cells. 54 Montgomery et al 34 have provided evidence that survival and proliferation of αvβ3-expressing melanoma cells in a three-dimensional collagen matrix is mediated through the ligation of the collagen proteolytic products to the cryptic binding site of αvβ3. Taken together, these findings suggest a role for the αvβ3 integrin in melanoma progression toward an increasingly aggressive phenotype.
In our study, tumorigenicity of early-stage melanoma cells was increased after overexpression of the β3 subunit. This finding contrasts with previous transfection studies that identified αv as the crucial component in conferring an aggressive phenotype 33 or that report decreased metastatic potential on β3 transduction. 35 The discrepancies might reflect a variability in the degree and aspect of phenotype modulation with the stage of progression, despite the profound effect of αvβ3 expression on the biological properties of melanoma cells. Indeed, metastatic cells are less susceptible than nontumorigenic RGP primary melanoma cells to alterations induced by αvβ3 overexpression. Thus, the biological functions of αvβ3 in melanoma may depend, in part, on the cellular background of a given stage of tumor progression.
Footnotes
Address reprint requests to Dr. Meenhard Herlyn, The Wistar Institute, 3601 Spruce Street, Philadelphia, PA 19104-4268. E-mail: herlynm@wista.wistar.upenn.edu.
Supported by NIH National Cancer Institute grants CA-47159 and CA-10815.
D.-T. Shih’s current address: Department of Biology, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT 06877.
J.-Y. Hsu’s current address: Sidney Kimmel Cancer Center, San Diego, CA 92121.
References
- 1.Clark WH, Jr, Elder DE, Guerry D, Epstein MN, Greene MH, Van Horn M: A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 1984, 15:1147-1165 [DOI] [PubMed] [Google Scholar]
- 2.Clark WH, Jr: Tumor progression and the nature of cancer. Br J Cancer 1991, 64:631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark WH, Jr, Elder DE, Guerry D, IV, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC: Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 1989, 82:626-627 [DOI] [PubMed] [Google Scholar]
- 4.Herlyn M, Thurin J, Balaban G, Bennicelli JL, Herlyn D, Elder DE, Bondi E, Guerry D, Nowell P, Clark WH, Koprowski H: Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res 1985, 45:5670-5676 [PubMed] [Google Scholar]
- 5.Herlyn M, Balaban G, Bennicelli J, Guerry D, Halaban R, Herlyn D, Elder DE, Maul GG, Steplewski Z, Nowell PC, Clark WH, Jr, Koprowski H: Primary melanoma cells of the vertical growth phase: similarities to metastatic cells. J Natl Cancer Inst 1985, 74:283-289 [PubMed] [Google Scholar]
- 6.Herlyn M, Clark WH, Rodeck U, Mancianti ML, Jambrosic J, Koprowski H: Biology of tumor progression in human melanocytes. Lab Invest 1987, 56:461-474 [PubMed] [Google Scholar]
- 7.Satyamoorthy K, Dejesus E, Linnenbach A, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M: Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res 1997, 7:S35-S42 [PubMed] [Google Scholar]
- 8.Hsu M-Y, Elder DE, Herlyn M: The Wistar melanoma (WM) cell lines. Human Cell Culture, vol. 3: Solid Cancers. Edited by Masters JRW, Palsson B. Kluwer Academic Publishers, Norwell, MA, 1998 (in press)
- 9.Shih I-M, Elder DE, Hsu M-Y, Herlyn M: Regulation of MelCAM/MUC18 expression on melanocytes of different stages of tumor progression by normal keratinocytes. Am J Pathol 1994, 145:837-845 [PMC free article] [PubMed] [Google Scholar]
- 10.Rodeck U, Herlyn M, Menssen HD, Furlanetto RW, Koprowski H: Metastatic but not primary melanoma cells grow in vitro independently of exogenous growth factors. Int J Cancer 1987, 40:687-690 [DOI] [PubMed] [Google Scholar]
- 11.Rodeck U, Melber K, Kath R, Menssen H-D, Varello M, Atkinson B, Herlyn M: Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J Invest Dermatol 1991, 97:20-26 [DOI] [PubMed] [Google Scholar]
- 12.Kath R, Jambrosic J, Holland L, Rodeck U, Herlyn M: Development of invasive and growth factor-independent cell variants from primary melanomas. Cancer Res 1991, 51:2205-2211 [PubMed] [Google Scholar]
- 13.Kath R, Rodeck U, Parmiter A, Jambrosic J, Herlyn M: Growth factor independence in vitro of primary melanoma cells from advanced but not early or intermediate lesions. Cancer Ther Control 1990, 1:179-191 [Google Scholar]
- 14.Hyne RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 15.Juliano R: Cooperation between soluble factors and integrin-mediated cell anchorage in the control of cell growth and differentiation. Bioessays 1996, 18:911-917 [DOI] [PubMed] [Google Scholar]
- 16.Giancotti FG: Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 1997, 9:691-700 [DOI] [PubMed] [Google Scholar]
- 17.Frisch SM, Ruoslahti E: Integrins and anoikis. Curr Opin Cell Biol 1997, 9:701-706 [DOI] [PubMed] [Google Scholar]
- 18.Danen EH, Van Muijen GN, Ruiter DJ: Role of integrins as signal transducing cell adhesion molecules in human cutaneous melanoma. Cancer Surv 1995, 24:43-65 [PubMed] [Google Scholar]
- 19.Mortarini R, Anichini A: From adhesion to signalling: roles of integrins in the biology of human melanoma. Melanoma Res 1993, 3:87-97 [PubMed] [Google Scholar]
- 20.De Wit PE, Van Muijen GN, De Waal RM, Ruiter DJ: Pathology of malignant melanoma, including new markers and techniques in diagnosis and prognosis. Curr Opin Oncol 1996, 8:143-151 [DOI] [PubMed] [Google Scholar]
- 21.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA: Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res 1990, 50:6757-6764 [PubMed] [Google Scholar]
- 22.Danen EH, Ten Berge PJ, Van Muijen GN, Van’t Hof-Grootenboer AE, Brocker EB, Ruiter DJ: Emergence of α5β1 fibronectin- and αvβ3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology 1994, 24:249-256 [DOI] [PubMed] [Google Scholar]
- 23.Danen EH, Jansen KF, Van Kraats AA, Cornelissen IM, Ruiter DJ, Van Muijen GN: αv-integrins in human melanoma: gain of αvβ3 and loss of αvβ5 are related to tumor progression in situ but not to metastatic capacity of cell lines in nude mice [published erratum appears in Int J Cancer 1995, 62:365]. Int J Cancer 1995, 61:491-496 [DOI] [PubMed] [Google Scholar]
- 24.Natali PG, Nicotra MR, Di Filippo F, Bigotti A: Expression of fibronectin, fibronectin isoforms and integrin receptors in melanocytic lesions. Br J Cancer 1995, 71:1243-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hieken TJ, Farolan M, Ronan SG, Shilkaitis A, Wild L, Das Gupta TK: β3 integrin expression in melanoma predicts subsequent metastasis. J Surg Res 1996, 63:169-173 [DOI] [PubMed] [Google Scholar]
- 26.Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S: Clinical significance of αvβ3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res 1997, 57:1554-1560 [PubMed] [Google Scholar]
- 27.Schadendorf D, Gawlik C, Haney U, Ostmeier H, Suter L, Czarnetzki BM: Tumour progression and metastatic behaviour in vivo correlates with integrin expression on melanocytic tumours. J Pathol 1993, 170:429-434 [DOI] [PubMed] [Google Scholar]
- 28.Gehlsen KR, Davis GE, Sriramarao P: Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis 1992, 10:111-120 [DOI] [PubMed] [Google Scholar]
- 29.Nip J, Rabbani SA, Shibata HR, Brodt P: Coordinated expression of the vitronectin receptor and the urokinase-type plasminogen activator receptor in metastatic melanoma cells. J Clin Invest 1995, 95:2096-2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JF, Nesbitt SA, Helfrich MH, Horton MA, Polakova K, Hart IR: Integrin expression in human melanoma cell lines: heterogeneity of vitronectin receptor composition and function. Int J Cancer 1991, 49:924-931 [DOI] [PubMed] [Google Scholar]
- 31.Seftor REB, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJC: Role of the αvβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA 1992, 89:1557-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitjans F, Sander D, Adan J, Sutter A, Martinez JM, Jaggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL: An anti-αv-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci 1995, 108:2825-2838 [DOI] [PubMed] [Google Scholar]
- 33.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA: Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Invest 1992, 89:2018-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery AM, Reisfeld RA, Cheresh DA: Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA 1994, 91:8856-8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danen EH, van Kraats AA, Cornelissen IM, Ruiter DJ, van Muijen GN: Integrin β3 cDNA transfection into a highly metastatic αvβ3-negative human melanoma cell line inhibits invasion and experimental metastasis. Biochem Biophys Res Commun 1996, 226:75-81 [DOI] [PubMed] [Google Scholar]
- 36.Boyce ST, Ham R: Cultivation, frozen storage, and clonal growth of human epidermal keratinocytes in serum free medium. J Tissue Culture Methods 1985, 9:83-93 [Google Scholar]
- 37.Graham FL, Prevec L: Methods for construction of adenovirus vectors. Mol Biotechnol 1995, 3:207-220 [DOI] [PubMed] [Google Scholar]
- 38.Brass L, Shatil S, Kunicki T, Bennet J: Effect of calcium on the stability of the platelet membrane glycoprotein IIb/IIIa complex. J Biol Chem 1985, 260:7875-7881 [PubMed] [Google Scholar]
- 39.Bell E, Sher S, Hull B, Merrill C, Rosen S, Chamson A, Asselineau D, Dubertret L, Coulomb B, Lapiere C, Nusgens B, Neveux Y: The reconstitution of living skin. J Invest Dermatol 1983, 81:2s-10s [DOI] [PubMed] [Google Scholar]
- 40.Asselineau D, Bernard BA, Bailly C, Darmon M: Retinoic acid improves epidermal morphogenesis. Dev Biol 1989, 133:322-335 [DOI] [PubMed] [Google Scholar]
- 41.Chen CS, Lyons-Giordano B, Lazarus GS, Jensen PJ: Differential expression of plasminogen activators and their inhibitors in an organotypic skin coculture system. J Cell Sci 1993, 106:45-53 [DOI] [PubMed] [Google Scholar]
- 42.Meredith JEJ, Fazeli B, Schwartz MA: The extracellular matrix as a cell survival factor. Mol Biol Cell 1993, 4:953-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 44.Boudreau N, Sympson CJ, Werb Z, Bissell M: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267:891-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varner JA, Cheresh DA: Integrins and cancer. Curr Opin Cell Biol 1996, 8:724-730 [DOI] [PubMed] [Google Scholar]
- 46.Seftor RE, Seftor EA, Stetler-Stevenson WG, Hendrix MJ: The 72 kd type IV collagenase is modulated via differential expression of αvβ3 and α5β1 integrins during human melanoma cell invasion. Cancer Res 1993, 53:3411-3415 [PubMed] [Google Scholar]
- 47.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 48.Gouon V, Tucker GC, Kraus-Berthier L, Atassi G, Kieffer N: Up-regulated expression of the β3 integrin and the 92-kd gelatinase in human HT-144 melanoma cell tumors grown in nude mice. Int J Cancer 1996, 68:650-662 [DOI] [PubMed] [Google Scholar]
- 49.Hillis GS, MacLeod AM: Integrins and disease. Clin Sci 1996, 91:639-650 [DOI] [PubMed] [Google Scholar]
- 50.Vuori K, Ruoslahti E: Association of insulin receptor substrate-1 with integrins. Science 1994, 266:1576-1578 [DOI] [PubMed] [Google Scholar]
- 51.Schneller M, Vuori K, Ruoslahti E: αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J 1997, 16:5600-5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boukerche H, Benchaibi M, Berthier-Vergnes O, Lizard G, Bailly M, Bailly M, McGregor JL: Two human melanoma cell-line variants with enhanced in vivo tumor growth and metastatic capacity do not express the β3 integrin subunit. Eur J Biochem 1994, 220:485-491 [DOI] [PubMed] [Google Scholar]
- 53.Shih I-M, Speicher DW, Hsu M-Y, Levine E, Herlyn M: Melanoma cell-cell interactions are mediated through heterophilic Mel-CAM/ligand adhesion. Cancer Res 1997, 57:3835-3840 [PubMed] [Google Scholar]
- 54.Deryugina EL, Bourdon MA, Luo G-X, Reisfeld RA, Strongin A: Matrix metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci 1997, 110:2473-2482 [DOI] [PubMed] [Google Scholar]