Abstract
Glioblastomas are highly vascular tumors which overexpress the angiogenesis factor vascular endothelial growth factor (VEGF). VEGF and its receptors, VEGF-R1 and VEGF-R2, have been shown to be necessary for embryonic angiogenesis as well as for tumor angiogenesis. Recently, the angiopoietin/Tie2 receptor system has been shown to exert functions in the cardiovascular system that are distinct from VEGF but are also critical for normal vascular development. To assess the potential role of Tie2 and its ligands angiopoietin-1 and angiopoietin-2 in tumor vascularization, we analyzed their expression pattern in human gliomas. Tie-2 was up-regulated in tumor endothelium compared to normal human brain tissue. We further observed cell type-specific up-regulation of the message for both angiopoietin-1 and angiopoietin-2 in gliomas. Whereas Ang-1 mRNA was expressed in tumor cells, Ang-2 mRNA was detected in endothelial cells of a subset of glioblastoma blood vessels. Small capillaries with few periendothelial support cells showed strong expression of Angiopoietin-2, whereas larger glioblastoma vessels with many periendothelial support cells showed little or no expression. Although the function of Tie2 and its ligands in tumor angiogenesis remains a subject of speculation, our findings are in agreement with a recently proposed hypothesis that in the presence of VEGF, local production of Ang-2 might promote angiogenesis.
Glioblastomas are the most common and most malignant brain tumor in humans. They arise either de novo (primary glioblastoma) or by progression from low-grade gliomas (secondary glioblastoma). Both primary and secondary glioblastomas are characterized by endothelial cell proliferation and prominent vascularization and can be considered model tumors for investigating angiogenesis associated with tumor progression. 1,2
Several studies 3 suggest that solid tumor growth to a clinically relevant size depends on adequate blood supply. Solid tumors recruit blood vessels from the neighboring tissue by angiogenesis, eg, the sprouting of capillaries from pre-existing vessels that migrate into the tumor and form a new vascular network. To stimulate angiogenesis, tumors secrete growth factors that act on endothelial cells. It is thought that the resulting neovasculature supports tumor expansion and metastasis.
According to gene targeting studies in mice, vascular endothelial growth factor (VEGF) and its tyrosine kinase receptors VEGF-R1 (flt-1) and VEGF-R2 (flk-1/KDR) are major regulators of angiogenesis and vasculogenesis in the developing embryo. 4 Mice deficient for VEGF die at embryonic day 8.5–9.0 by impairment of both vasculogenesis and angiogenesis. 5,6 Disruption of the VEGF-R2 gene interferes with endothelial cell differentiation and causes embryonic death on embryonic day 8.5. 7 Disruption of the VEGF-R1 gene permits differentiation of endothelial cells but results in phenotypically abnormal, disorganized blood vessels leading to death of embryos at embryonic day 9.0. 8 In addition to embryonic angiogenesis, VEGF and its receptors are also key regulators of tumor angiogenesis. VEGF mRNA is highly up-regulated in hypoxic tumor cells in human and rat glioblastomas, while VEGF receptors are up-regulated in tumor endothelial cells. 9-13 A role for the VEGF/VEGF receptor system in tumor angiogenesis has been proven by several experimental approaches using anti-VEGF antibodies, 14,15 VEGF-antisense cDNA, 16,17 dominant-negative VEGF-R2 mutants, 18-20 anti-VEGF-R2 antibodies, 21 and soluble VEGF-R2. 22 Using different animal models, all approaches resulted in significant inhibition of tumor angiogenesis.
Tie1 and Tie2 (tek) are members of the only other known endothelial cell-specific receptor tyrosine kinase family. 23-25 Like VEGF and its receptors, Tie1 and Tie2 are essential for the formation of embryonic vasculature. Transgenic mice deficient for Tie1 or Tie2 showed vascular defects such as edema and localized hemorrhage (Tie1 knockout), 26,27 dilated blood vessels, absence of capillary sprouts, and an abnormal vascular network (Tie2 knockout), 27,28 resulting in embryonic or early postnatal death. In contrast to VEGF signaling, which is essential for endothelial cell differentiation, Tie1 and Tie2 seem to be important for vascular remodeling and sprouting. 28 In contrast to the established role of Tie1 and Tie2 in embryonic angiogenesis and vessel maturation, their role in blood vessel growth associated with tumor development remains unclear. It has been shown that Tie1 is up-regulated in the vascular endothelium of metastatic melanomas, 29 brain tumors, 30 and mammary carcinomas. 31 The potential importance of Tie2 in pathological vascular growth was suggested by inhibition studies using soluble extracellular domains of Tie2. 32
A ligand for the Tie1 receptor has not yet been found but the newly discovered ligand for the Tie2 receptor, Angiopoietin-1 (Ang-1), has recently been shown to be essential for normal vascular development in the mouse. 33,34 Its naturally occurring antagonist Angiopoietin-2 (Ang-2) binds with similar affinity to Tie2 but, unlike Ang-1, which induced receptor phosphorylation on ligand binding, Ang-2 blocked Tie2 activity. 35 The absence of Ang-1 caused severe vascular abnormalities in the developing mouse embryo including a less complex vascular network with fewer branches and dilated vessels. 34 Transgenic overexpression of Ang-2 revealed malformation of the vascular network and widespread vessel discontinuities in the embryo, defects similar to those seen in mice lacking Ang-1 or Tie2. 35 Based on these studies, it has been proposed that the angiopoietin/Tie2 receptor system plays a role in the interaction of endothelial cells with smooth muscle cells/pericytes (SMC/PC). 36 Pericytes have been shown to inhibit endothelial cell proliferation in vitro and are thought to be an important regulator of blood vessel growth in vivo. 37
To define a putative role for the Tie2/angiopoietin system in glioblastoma angiogenesis, we examined expression and cellular distribution of Ang-1 and Ang-2 mRNA by Northern blot analysis and in situ hybridization, and of Tie2 by immunohistochemistry. We observed cell type-specific up-regulation of Tie2 and its ligands Ang-1 and Ang-2 during tumor progression in a pattern compatible with a role in tumor-induced angiogenesis.
Materials and Methods
Tissue Specimens
Fourteen glioma specimens (one pilocytic astrocytoma WHO grade I, four astrocytomas WHO grade II, two anaplastic astrocytomas WHO grade III, and seven glioblastomas WHO grade IV) were freshly received from the neurosurgical theater, directly frozen in liquid nitrogen, and stored at −80°C before use. Normal brain and cerebellar tissue from two patients without neurological disease were also snap-frozen. The frozen tissue was cryosectioned for immunohistochemistry and in situ hybridization. Part of the material was used for extraction of total RNA.
For in situ hybridization frozen sections (10 μm thick) from TissueTek (Miles, Elkhart, IN) embedded tumor tissue were melted on silan-covered (TESPA, 3-aminopropyl-triethoxy-silane) glass slides. Two serial sections were put on each slide and used for antisense and sense (control) hybridization. Sections were fixed in 4% paraformaldehyde and dehydrated in a series of 30%, 60%, 80%, 95%, and 100% ethanol. After air-drying slides were stored at −80°C until used for in situ hybridization.
For immunohistochemistry cryostat sections (10 μm) were air-dried and acetone-fixed. Sections were stored at −20°C before use.
Immunohistochemistry
Slides were washed in phosphate-buffered saline (PBS) and incubated for 30 minutes in 5% bovine serum albumin, Fraction V (Sigma, Deisenhofen, Germany) in PBS. Sections were then incubated with primary antibody for 1 hour at room temperature. Primary antibodies used were monoclonal antibodies against human CD31 (dilution 1:40, Dako, Geostrup, Demnark), against α smooth muscle actin (dilution 1:300, Sigma), and against human Tie2 38 (10 μg/ml, gift of Dr. Kevin Peters, Durham, NC), and polyclonal antibodies against human von Willebrand factor (1:1000, Sigma). To demonstrate specificity of Tie2 immunohistochemistry, 10μg/ml of antibody were preincubated with 230 μg/ml recombinant extracellular tie2-Fc 35 (gift of Dr. G. D. Yancopoulos, Tarrytown, NY) for 15 minutes at room temperature. After washing with PBS, 0.1% Triton-X-100 and blocking with 20% normal goat serum (Dianova, Hamburg, Germany) in PBS for 30 minutes, slides were incubated with secondary antibody for 1 hour at room temperature (biotinylated goat anti-mouse IgG, 1:300 or biotinylated goat anti-rabbit IgG, 1:300). For blocking of endogenous peroxidase activity sections were incubated in 0.3% H2O2 in methanol for 30 minutes at 4°C. Finally, sections were incubated in a streptavidin-peroxidase-complex (Vectastain, Vector, Burlingame, CA) according to the manufacturer’s protocol. Color detection of immunoreactivity was achieved using AEC (3-amino-9-ethyl-carbazole, Sigma, 0.26 mg/ml in acetate buffer, pH 5.2) and 0.03% H2O2 as a substrate for peroxidase. Color reaction time was 10–30 minutes at room temperature.
Isolation of Total RNA and Northern Blotting
Total cytoplasmic RNA was isolated from 100 mg of tissue by the guanidinium-isothiocyanate method. 39 Aliquots of 10–12 μg RNA were electrophoresed on a 1.4% agarose gel containing 5.5% formaldehyde in 1× MOPS buffer (20 mmol/L 3-(n-morpholino) propane sulfonic acid, pH 7.0, 8 mmol/L sodium acetate, 1 mmol/L EDTA) and transferred to a nylon membrane (Duralon, Stratagene, Heidelberg, Germany) in 20× standard saline citrate (SSC) buffer. RNA was UV-crosslinked to the membrane (120 mJ/cm2) and filters were hybridized with [32P]dCTP-labeled cDNA probe (Prime-It II random primer labeling kit, Stratagene). Human Ang-cDNA templates described in the next paragraph were used for random priming. Hybridization in QuikHyb hybridization solution (Stratagene) plus 200 mg/ml salmon sperm DNA was for 1 hour at 68°C. Two stringency washes in 2× SSC, 0.1% sodium dodecyl sulfate (SDS) at 60°C for 10 minutes were followed by a final wash in 0.1× SSC, 0.1% SDS at 60°C for 5 minutes. Blots were exposed to Kodak X-OMAT-XAR-2 films with an intensifying screen at −70°C for 2–10 days.
RNA Probe Generation by in Vitro Transcription
A 570-bp SpeI-EcoRI fragment from human Ang-1 cDNA subcloned into XbaI/EcoRI site of pBluescript KS+ containing 70 bp of 5′-untranslated region and 500 bp of coding region ending at amino acid 166 was used to generate nonradioactive antisense and sense RNA probes by in vitro transcription. After linearization of the plasmid, single-stranded runoff transcripts were synthesized from the T7/T3 polymerase promoters using Digoxigenin-labeled uridine triphosphate as a substrate according to the manufacturer’s instructions (Digoxigenin RNA labeling kit, Boehringer, Mannheim, Germany). RNA probes for human Ang-2 were transcribed from a 640-bp EcoRI-HindIII fragment from human Ang-2 cDNA containing 360 bp of 5′-untranslated region and 280 bp of coding region ending at amino acid 99 subcloned into EcoRI/HindIII site of pBluescript KS+. After determination of concentration and labeling efficiency, Digoxigenin labeled probes were stored at −20°C or used at once for in situ hybridization analysis. Sense probes served as a control.
Nonradioactive in Situ Hybridization
Prehybridization treatment consisted of 0.2 mol/L HCl for 5 minutes followed by digestion with Proteinase K (10 μg/ml) for 10 minutes at room temperature. After postfixation with 4% paraformaldehyde sections were acetylated with 0.1 mol/L triethanolamine and 0.25% acetic anhydride for 10 minutes at room temperature. Prehybridization solution containing 4× SSC, 50% deionized formamide, 2% SDS, 5× Denhardt’s solution, 10% dextran sulfate and 0.5 mg/ml of yeast tRNA was applied for 1.5 hours at 48°C. RNA sense/antisense probe concentration for hybridization was 0.1–0.5 ng/μl of initial transcript. Tissue sections were incubated in a humidified chamber under glass coverslips at 48°C overnight. Posthybridization stringency washes at 48°C included 2× SSC for 30 minutes, 2× SSC plus 0.1% SDS for 5 minutes, 0.1× SSC plus 0.1% SDS for 15 minutes. Each wash was carried out twice. After RNase A treatment (2.5 μg/ml in 2× SSC) for 5 minutes at 37°C, hybridized probes were detected by anti-DIG antibody conjugated to alkaline phosphatase (Boehringer) diluted 1:500 for 1 hour at room temperature. Nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution was used as color substrate in 0.1 mol/L Tris/Cl, 0.1 mol/L NaCl, pH 9.5. Color reaction times ranged from 14 to 20 hours, after which slides were rinsed in TE (10 mmol/L Tris/Cl, 1 mmol/L EDTA, pH 8.0) and mounted in glycerol.
In Situ Hybridization/Immunohistochemistry Double Labeling Analysis
To identify Ang-2 mRNA expressing cells, a double labeling technique combining in situ hybridization and immunohistochemistry was applied. Slides were subjected to the in situ hybridization procedure described above and then directly transferred into PBS. Subsequent immunohistochemistry with anti-van Willebrand factor and anti-smooth muscle actin antibodies was performed following the above protocol but omitting the hydrogen peroxide/methanol blocking step.
Results
Five neurosurgically removed low-grade (WHO grades I and II) and nine high-grade (WHO grades III and IV) gliomas and two normal brain specimens were analyzed by Northern blotting and in situ hybridization for Ang-1 and Ang-2 expression and by immunhistochemistry for Tie2 expression. Tie2 was expressed in vascular endothelial cells of normal brain but was up-regulated in endothelial cells of tumors (Figure 1) ▶ . In all tissue specimens, Tie2 expression was confined to endothelial cells and neither normal neuroectodermal cells nor glioma cells expressed Tie2. Smooth muscle actin was strongly expressed in periendothelial cells (probably smooth muscle cells and pericytes) but not in endothelial cells or tumor cells. Small capillaries were associated with single SMA-positive cells, but larger tumor vessels showed an abundance of SMA-positive cells when compared to vessels of similar size in normal brain.
Figure 1.
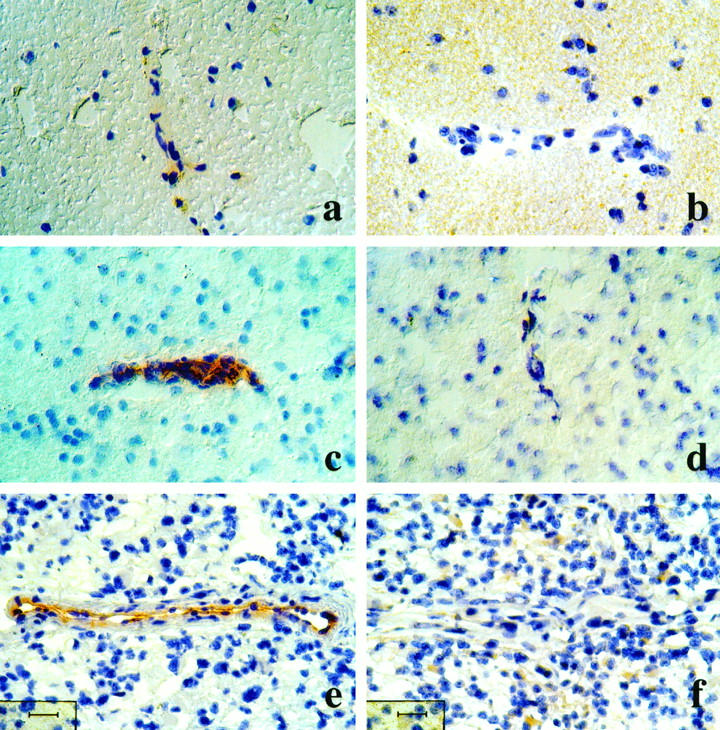
Immunohistochemistry for Tie2 in normal brain (a,b), astrocytoma (c,d), and glioblastoma (e,f). a, c, and e show immunoreactivity for Tie2. b, d and f represent control experiments with blocked antibody. Magnification, ×400; scale = 10 μm.
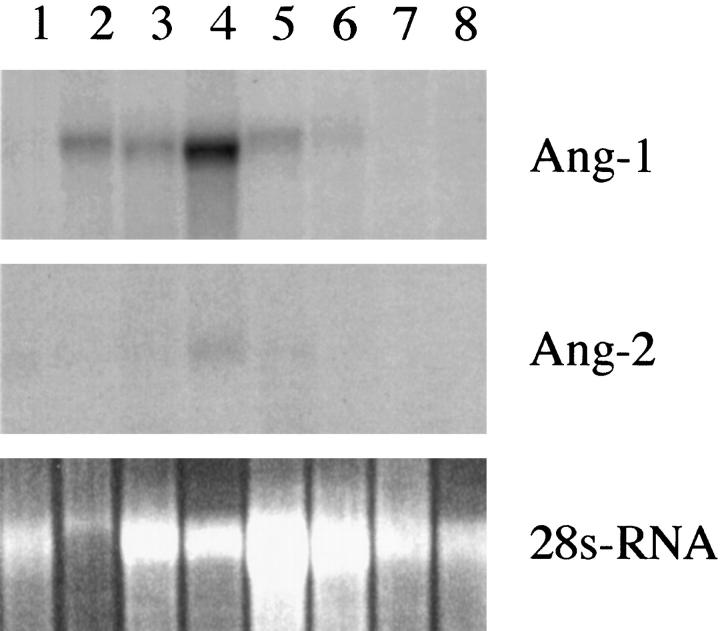
By Northern blot analysis we observed up-regulation of the activating Tie2 ligand, Ang-1, in the glioma specimens (Figure 2) ▶ . Whereas in normal brain and low-grade gliomas no or faint signals were observed, Ang-1 mRNA expression was clearly detectable in high-grade gliomas. Like Ang-1, Ang-2 mRNA was not observed in normal brain but its expression was seen in glioblastoma specimens, albeit at a lower level.
Figure 2.
Northern blot analysis of Ang-1 and Ang-2 mRNA expression in normal brain, low-grade gliomas, and high-grade gliomas. Total RNA samples of 10 μg prepared from normal brain (lane1), three glioblastomas WHO grade IV (lanes 2–4), one anaplastic astrocytoma WHO grade III (lane 5), two astrocytomas WHO grade II (lanes 6–7), and one pilocytic astrocytoma WHO grade I (lane 8) were probed for the indicated transcripts. Ethidium bromide-stained gel indicates amount of RNA in each lane.
By in situ hybridization transcripts for Ang-1 were observed in neuronal cells of cerebrum and cerebellum. Expression was also observed in vascular cells (Figure 3a) ▶ . In glioblastoma, Ang-1 was expressed in almost all tumor cells. Areas of more intense staining alternated with areas of less intense or very weak staining. No focal up-regulation of angiopoietin-1, which has been found to be characteristic for hypoxia-induced VEGF expression in glioblastomas, was observed (Figure 3c) ▶ . Ang-2 expression in normal brain was observed in neurons such as cerebellar Purkinje’s cells. No expression was seen in vascular cells (Figure 3e) ▶ . In glioblastoma, Ang-2 transcripts were not observed in tumor cells but found associated with tumor vessels (Figure 3g) ▶ . However, Ang-2 transcripts were restricted to a subset of blood vessels in a given section. Smaller vessels and capillaries were stained for Ang-2 mRNA (Figure 3i) ▶ , whereas larger vessels showed only weak staining or were completely negative (Figure 3j) ▶ .
Figure 3.
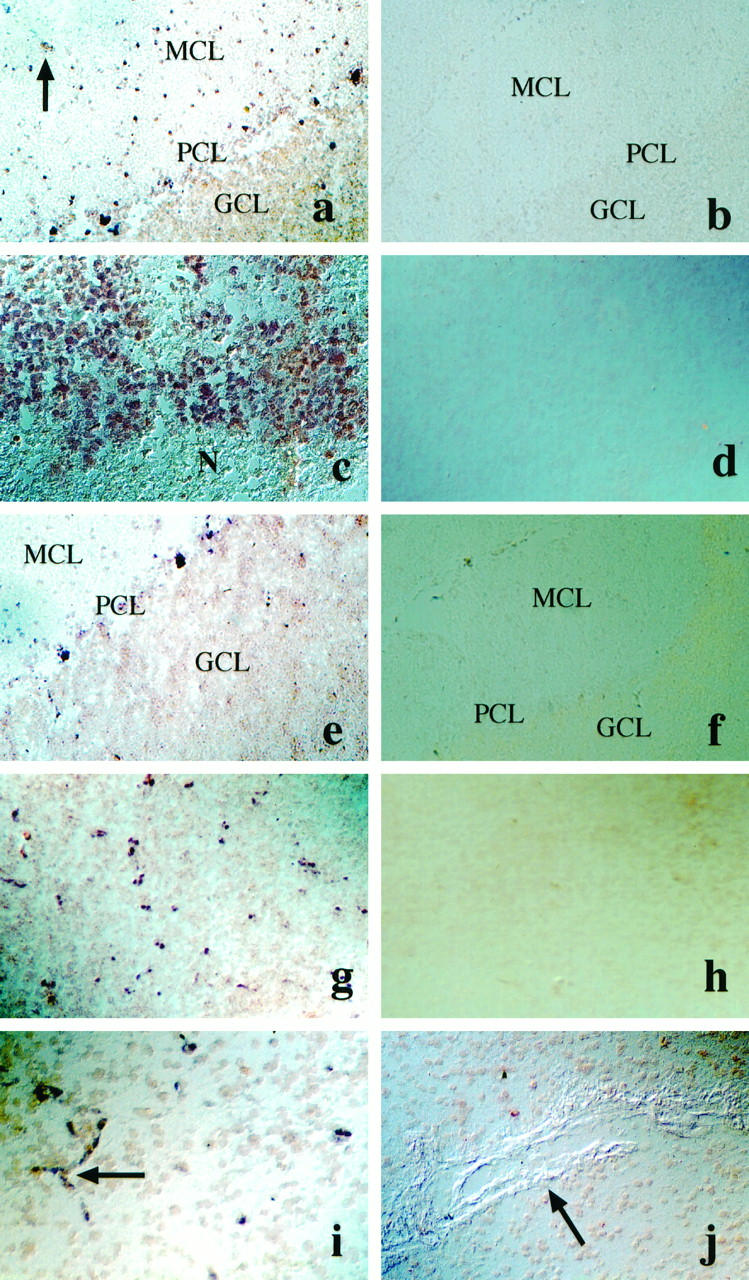
In situ hybridization analysis of Ang-1 and Ang-2 mRNA expression in human brain and in glioblastoma. a-d: Ang-1 in situ hybridization in cerebellum (a) and in glioblastoma (c). Arrow in a denotes blood vessel expressing Ang-1. b and d: Ang-1 sense-control hybridization. Magnification, ×100. e-f: Ang-2 in situ hybridization in cerebellum (e) and in glioblastoma (g, i, j). g and i: Ang-2 expression in small vessels. Arrowhead in (i) denotes vessel sprout, (j) no Ang-2 expression in large vessel. f and h represent Ang-2 sense control hybridization. Magnification, ×100 (e-i); ×200 (j). MCL, molecular cell layer; PCL, Purkinje’s cell layer; GCL, granular cell layer; N, necrosis
To further identify the Ang-2 expressing cell type in tumor blood vessels we performed a double labeling analysis combining in situ hybridization and immunohistochemistry approaches. Sections were first subjected to Ang-2 in situ hybridization followed immediately by immunohistochemistry with antibodies against vWF. Ang-2 mRNA and endothelial cell-specific vWF protein were found to be exactly colocalized (Figure 4c,d) ▶ . We therefore conclude that Ang-2 is expressed in endothelial cells of glioblastoma vessels. vWF stained endothelial cells of both smaller capillaries and large vessels. Colocalization of Ang-2 and vWF protein was observed only in endothelial cells of smaller vessels, whereas endothelial cells of large vessels only expressed vWF (Figure 4 ▶ c).
Figure 4.
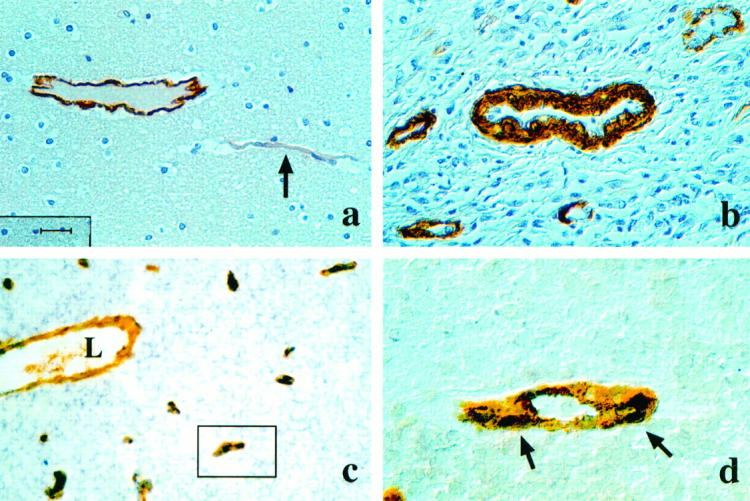
Smooth muscle actin expression in normal brain (a) and in glioblastoma (b). a: Few SMA-positive cells in postcapillary venule (arrow). b: Extensive recruitment of SMA-positive cells to the tumor vasculature in glioblastoma. Magnification, ×200 (a), (scale = 20 μm); ×400 (b). c,d: Double labeling in situ hybridization (ISH) and immunohistochemistry (IHC) for Ang-2 mRNA and vWF expression in glioblastoma. Ang-2 mRNA is expressed in a subset of vWF-positive endothelial cells (c). Note overlapping ISH and IHC staining for Ang-2 (dark brown) and vWF (orange). L: Lumen of large vessel with vWF-positive endothelial cells not expressing Ang-2. Magnification, ×100. (d) Blow-up of vessel marked in (c). Ang-2 mRNA expression (dark brown staining, arrows) is localized in vWF-positive (orange staining) vascular endothelial cell. Magnification, ×630.
Discussion
Ang-1 and Ang-2 are secreted glycoproteins of approximately 75 kd with approximately 60% identity in amino acid sequence. Both Ang-1 and Ang-2 bind to Tie2 with similar affinity but have different effects on the receptor. Whereas Ang-1 induced autophosphorylation of the receptor in cultured endothelial cells, Ang-2 competitively inhibited Ang-1-induced kinase activation of Tie2. 35
To assess the potential role of Ang-1 and Ang-2 in glioma vascularization, we analyzed their expression in normal brain and in low- and high-grade gliomas. We observed cell type-specific up-regulation of the mRNA for both angiopoietins during glioma progression. Ang-1 was expressed only weakly in low-grade gliomas, but showed strong expression in high-grade gliomas. In glioblastomas, Ang-1 mRNA was expressed in almost all tumor cells. No focal up-regulation in certain tumor areas, typically observed for VEGF mRNA in perinecrotic palisading cells, was found for Ang-1 in the tumor sections analyzed. This finding is consistent with the observation that unlike VEGF mRNA, Ang-1 mRNA is not up-regulated by hypoxia. 40 Ang-2 was expressed in vascular cells of high-grade gliomas, but not in vessels of low-grade gliomas and normal brain. Although vascular expression of Ang-2 has been described previously, the exact cell type has not been defined. From previous experiments in E12.5 mice, 34 it is known that Ang-2 transcripts are associated with vascular structures such as the smooth muscle cell layer of the dorsal aorta and major aortic branches. In fetal liver Ang-2 mRNA was located at or close to the lumen of hepatic vessels in so-called endothelial-like cells. Ang-2 mRNA, however, was observed in some but not all hepatic vessels. Because it was difficult to determine whether in our glioblastoma sections Ang-2 mRNA was expressed in endothelial or periendothelial cells, we applied a double-labeling technique consisting of nonradioactive in situ hybridization to detect Ang-2 mRNA combined with immunohistochemical detection of endothelial (vWF) and smooth muscle/pericyte proteins (SMA) on the same tissue section. With this technique tumor endothelial cells were unequivocally identified as Ang-2 mRNA-expressing cells. Interestingly, the overlap of Ang-2 mRNA and vWF protein was confined to smaller vessels, whereas in larger vessels no Ang-2 expression could be observed in endothelial cells.
We suspect that endothelial Ang-2 expression characterizes angiogenic glioblastoma blood vessels. Because Ang-1 is thought to be important for stabilizing the vessel wall, local Ang-2 expression might promote smooth muscle cell/pericyte dropoff, which is thought to be a requirement for rendering and maintaining endothelial cells accessible to angiogenic inducers. In glioblastomas, this angiogenic signal most likely is VEGF, which has been shown to be necessary for glioma angiogenesis. 14,16-18 Absence of Ang-2 in larger glioblastoma vessels was negatively correlated with the number of SMC/PC because endothelial cells in larger vessels were associated with many more SMA-expressing cells than those in smaller vessels. It has recently been proposed that the Angiopoietin/Tie2 system might regulate the interaction of endothelial cells with surrounding mesenchymal cells. 41 This model is based on ultrastructural analysis in Ang-1 (−/−) mouse embryos that revealed a defect in smooth muscle cell and pericyte precursor cell recruitment, resulting in an abnormal vasculature. 34 Because a similar phenotype was observed in Ang-2-overexpressing mice, 35 it was suggested that a physiological role of Ang-1 could be to recruit periendothelial cells and support the physical association of endothelial cells with these cells. According to this model, inhibition of Ang-1 by Ang-2 drives angiogenesis in the presence of angiogenesis inducers such as VEGF by loosening contacts between endothelial and periendothelial cells. 41 Although this hypothesis remains speculative, our findings in human gliomas favor this model.
Glioblastomas are highly angiogenic tumors that overexpress VEGF and platelet-derived growth factor-B (PDGF-B) chain. 9,10,12,42 Whereas VEGF is a specific mitogen for vascular endothelial cells, PDGF-B stimulates the proliferation of mesenchymal cells, including smooth muscle cells and pericytes. 43 Glioblastoma blood vessels typically consist of endothelial cells covered by multiple layers of SMC/PC. 44,45 Whereas endothelial cells express PDGF-B and VEGF-R1 and-R2, SMC/PC express PDGF-Rβ. 1,11,42,46 It is therefore likely that in glioblastomas endothelial cell proliferation is driven by tumor-derived VEGF, whereas SMC/PC proliferation is driven by endothelium-derived (and probably also tumor-derived) PDGF-B. Our observation that endothelial Ang-2 expression in glioblastomas is limited to small vessels with few SMC/PC, whereas larger vessels with many SMC/PC did not express Ang-2, supports the hypothesis that Ang-2 promotes angiogenesis in situ. Down-regulation of Ang-2 in quiescent endothelial cells may permit physical interaction of endothelial cells with SMC/PC, leading to inhibition of endothelial cell proliferation and maturation of the vascular wall. This hypothesis is supported by angiopoietin expression observed in normal brain because in quiescent brain vessels Ang-1 but not Ang-2 was expressed. Thus, whereas in normal blood vessel Ang-1 expression exceeds Ang-2 expression, the opposite is true in tumor blood vessels. Ang-2 up-regulation in endothelial cells is therefore associated with tumor angiogenesis and may even be a prerequisite for the induction of tumor angiogenesis. In addition to its putative function in vascular remodeling, Ang-1 has been found to induce vascular sprouting in vitro. 47 Thus, it also appears possible that Ang-1 acts synergistically with Ang-2 to promote glioblastoma angiogenesis by inducing vascular sprouting.
Acknowledgments
We thank Dr. George D. Yancopoulos (Tarrytown, NY) for providing Ang-1 and Ang-2 cDNA clones and Tie2-Fc; Dr. Kevin Peters (Durham, NC) for the gift of the monoclonal anti-Tie2 antibody; Elife Iyen, Richard Haas, and Simone Erhardt for technical assistance; and Dr. Urban Deutsch (Bad Nauheim, Germany) for helpful discussions.
Footnotes
Address reprint requests to Dr. Karl H. Plate, Neurozentrum, Abteilung Neuropathologie, Breisacherstr. 64, 79106 Freiburg, Germany. E-mail: plate@nz11.ukl.uni-freiburg.de.
Supported by grant number W44/94/Ri 2 from the Deutsche Krebshilfe.
References
- 1.Kleihues P, Burger PC, Plate KH, Ohgaki H, Cavenee WK: Glioblastoma: Pathology and Genetics of Tumours of the Nervous System. Edited by Kleihues P, Cavenee WK. Lyon, France, International Agency for Research on Cancer, 1997, pp 16–24
- 2.Brem S, Cotran R, Folkman J: Tumor angiogenesis: a quantitative method for histological grading. J Natl Cancer Inst 1972, 48:347-356 [PubMed] [Google Scholar]
- 3.Folkman J: Tumor angiogenesis. Adv Cancer Res 1985, 43:175-203 [DOI] [PubMed] [Google Scholar]
- 4.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A: Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 383:73-75 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan K, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380:439-442 [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC: Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376:62-66 [DOI] [PubMed] [Google Scholar]
- 8.Fong GH, Rossant J, Gertsenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 9.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 10.Plate KH, Breier G, Risau W: Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol 1994, 4:207-218 [DOI] [PubMed] [Google Scholar]
- 11.Plate KH, Breier G, Weich HA, Mennel HD, Risau W: Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 1994, 59:520-529 [DOI] [PubMed] [Google Scholar]
- 12.Plate KH, Breier G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359:845-847 [DOI] [PubMed] [Google Scholar]
- 13.Plate KH, Breier G, Millauer B, Ullrich A, Risau W: Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res 1993, 53:5822-5827 [PubMed] [Google Scholar]
- 14.Kim KJ, Li B, Winer J, Armanini M, Gillet N, Philipps HS, Ferrara N: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 15.Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N: Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res 1996, 56:4032-4039 [PubMed] [Google Scholar]
- 16.Cheng SY, Huang HJS, Nagane M, Ji XD, Wang DG, Shih CCY, Arap W, Huang CM, Cavanee WK: Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA 1996, 93:8502-8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh M, Stacker SA, Wilks AF: Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence. Cancer Res 1996, 56:393-401 [PubMed] [Google Scholar]
- 18.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A: Glioblastoma growth inhibited in vivo by a dominant-negative flk-1 mutant. Nature 1994, 367:576-579 [DOI] [PubMed] [Google Scholar]
- 19.Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM: Dominant-negative inhibition of flk-1 suppresses the growth of many tumour types in vivo. Cancer Res 1996, 56:1615-1620 [PubMed] [Google Scholar]
- 20.Stratmann A, Machein MR, Plate KH: Anti-angiogenic gene therapy of malignant glioma. Acta Neurochir 1997, 68:105-110 [DOI] [PubMed] [Google Scholar]
- 21.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE: Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997, 3:1222-1227 [DOI] [PubMed] [Google Scholar]
- 22.Lin P, Sankar S, Shan S, Dewhirst MW, Polverini PJ, Quinn TQ, Peters KG: Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ 1998, 9:49-58 [PubMed] [Google Scholar]
- 23.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML: Tek, novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells, and their presumptive precursors. Oncogene 1992, 7:1471-1480 [PubMed] [Google Scholar]
- 24.Schnürch H, Risau W: Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial lineage. Development 1993, 119:957-968 [DOI] [PubMed] [Google Scholar]
- 25.Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G: Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene 1993, 8:1631-1637 [PubMed] [Google Scholar]
- 26.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J: The receptor tyrosine kinase tie is required for integrity and survival of vascular endothelial cells. EMBO J 1995, 14:5884-5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Quin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 28.Dumont DJ, Gradwohl G, Fong G-H, Puri MC, Gertsenstein M, Auerbach A, Breitman ML: Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994, 1909, 8:1897. [DOI] [PubMed] [Google Scholar]
- 29.Kaipainen A, Vlaykova T, Hatva E, Böhling T, Jekunen A, Pyrhonen S, Alitalo K: Enhanced expression of the tie receptor tyrosine kinase messenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res 1994, 54:6571-6577 [PubMed] [Google Scholar]
- 30.Hatva E, Kaipainen A, Jääskelainen J, Paetau A, Haltia M, Alitalo K: Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumours. Am J Pathol 1995, 146:368-378 [PMC free article] [PubMed] [Google Scholar]
- 31.Salven P, Joensuu H, Heikkila P, Matikainen MT, Wasenius VM, Alanko A, Alitalo K: Endothelial tie growth factor receptor provides angiogenic marker for assessment of breast cancer angiogenesis. Br J Cancer 1996, 74:69-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K: Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 1997, 100:2072-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the tie2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 [DOI] [PubMed] [Google Scholar]
- 34.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the tie2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 35.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 36.Folkman J, D’Amore PA: Blood vessel formation: what is its molecular basis? Cell 1996, 87:1153-1155 [DOI] [PubMed] [Google Scholar]
- 37.Orlidge A, D’Amore PA: Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 1987, 105:1455-1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG: Tie2 expression, and phosphorylation in angiogenic, and quiescent adult tissues Circ Res 1997, 81:567-574 [DOI] [PubMed] [Google Scholar]
- 39.Chomczynski P, Sacchi N: Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 40.Enholm B, Paavonen K, Ristimaki A, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14:2475-2483 [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D: Signaling vascular morphogenesis and maintenance. Science 1997, 277:48-50 [DOI] [PubMed] [Google Scholar]
- 42.Hermansson M, Nistér M, Betsholz C, Heldin CH, Westermark B, Funa K: Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci USA 1988, 85:7748-7752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato N, Beitz JG, Kato J, Yamamoto M, Clark JW, Calabresi P, Frackelton AR, Jr.: Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. Am J Pathol 1993, 142:1119-11130 [PMC free article] [PubMed] [Google Scholar]
- 44.Haddah SF, Moore SA, Schelper RL, Goeken JA: Vascular smooth muscle hyperplasia underlies the formation of glomeruloid vascular structures of glioblastoma multiforme. J Neuropath Exp Neurol 1992, 51:488-492 [DOI] [PubMed] [Google Scholar]
- 45.Wesseling P, Schlingenmann RO, Rietveld FJR, Link M, Burger PC, Ruiter DJ: Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropath Exp Neurol 1995, 54:304-310 [DOI] [PubMed] [Google Scholar]
- 46.Lindahl P, Johansson BR, Levéen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277:242-245 [DOI] [PubMed] [Google Scholar]
- 47.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W: Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol 1998, 8:529-532 [DOI] [PubMed] [Google Scholar]