Abstract
Current evidence suggests the papillary thyroid carcinoma oncogene (RET/PTC) generates papillary thyroid carcinomas in one genetic step. We tested a resulting prediction that RET/PTC expression in thyroid epithelium should be sufficient to cause the changes in nuclear morphology diagnostic of this tumor. Primary cultures of human thyroid epithelial cells were infected with a RET/PTC retroviral construct. Morphological scoring by two independent cytopathologists shows RET/PTC expression by immunohistochemistry to be highly associated (p ≪ 0.0001) with an irregular nuclear contour and a euchromatic appearance compared with non-expressing cells in the same cultures. The altered nuclear morphology is not due to gene transfer or transformation per se as primary thyroid cell cultures infected with a retroviral H-RAS construct differ from RET/PTC-infected cells by showing round nuclear envelopes and coarser chromatin, as determined by the independent scoring of two cytopathologists (p ≪ 0.0001). In addition, RET/PTC-transfected cells appear to disperse, whereas RAS-transfected cells grow as discrete colonies. The results provide additional support for the hypothesis that RET/PTC is sufficient to cause papillary thyroid carcinomas. A signaling pathway downstream of RET/PTC leads to restructuring of the nuclear envelope and chromatin, and the signal does not depend entirely, if at all, on a RAS pathway.
Thyroid epithelial cells can be transformed via two different pathways, a follicular pathway and a papillary pathway. The two types of resulting thyroid epithelial neoplasms are distinctive in their clinical features and morphology 1-3 as well as their genetics. 4-8 RAS mutations are common in the follicular pathway and appear to be an early event. 4,8 It seems likely that follicular carcinomas arise from follicular adenomas, although there is no direct supporting evidence. The defining feature of malignancy in the follicular pathway is vascular or capsular invasion. 1-3 Nuclear morphological features overlap considerably between follicular carcinomas, follicular adenomas, and even normal thyroid epithelium. 3,9,10
The papillary pathway is defined by a combination of morphological features, the most important of which are nuclear alterations: nuclear contour irregularities, such as nuclear grooves and inclusions, and dispersal of heterochromatin aggregates into a finely textured (ground-glass) chromatin. 1,3,11 Unlike most other solid tumors, including follicular thyroid carcinoma, there is no recognized benign counterpart of papillary thyroid carcinoma. Rather, papillary thyroid carcinomas appear histologically to arise in a single step; the unusual nuclear morphology diagnostic of this tumor can be identified within occult, relatively homogeneous foci composed of very few cells, 12 and such occult tumors do not appear to arise from a morphologically altered population.
The papillary thyroid carcinoma oncogene (RET/PTC) is a rearranged version of the tyrosine kinase RET, the receptor for glial-cell-line-derived neurotrophic factor. 13 RET/PTC rearrangement is nearly restricted to papillary thyroid carcinomas. No rearrangement of RET/PTC has ever been detected in more than 500 examples of nonthyroid tumors, 14 and RET/PTC rearrangements in nonpapillary thyroid lesions are restricted to a very small percentage of possible follicular-type lesions. 6,15,16 RET/PTC is activated when RET undergoes a translocation fusing its tyrosine kinase domain to a variety of other 5′ elements, thereby removing the promoter, the extracellular ligand-binding domain, and the membrane-anchoring domain of RET (reviewed in Refs. 7 and 17 ). RET is expressed in neural-crest-derived tissues, including thyroid C cells, but thyroid follicular cells express at most minimal amounts of RET mRNA. 18,19 In contrast, the 5′ sequences that donate their promoters and become fused to RET are all ubiquitously expressed, including in thyroid follicular epithelium. Three functional consequences of the translocations giving rise to RET/PTC could be significant for its transforming activity: 1) transcriptional activation driven by the new 5′ promoter, 2) oligomerization of RET, through motifs in the 5′ sequences, with resulting constitutive phosphorylation activity, 20-22 and 3) loss of membrane association due to deletion of the transmembrane-anchoring sequences of RET in the translocation. 7,23 RET/PTC is active in 25% to 80% of papillary thyroid carcinomas. 16 The incidence of RET/PTC rearrangements is highest in radiation-associated papillary thyroid carcinomas. 16,24,25 Papillary thyroid carcinomas expressing RET/PTC tend to be small and show classic morphology. 26
Genetic studies suggest that RET/PTC activation is an early genetic event. In keeping with the histological evidence that papillary thyroid carcinomas appear to arise in one genetic step, five pieces of evidence suggest that RET/PTC per se may be sufficient to mediate the transformation. 1) Occult papillary thyroid carcinomas, composed of very few cells, have been shown to sometimes express RET/PTC. 19 2) Immunohistochemistry of RET/PTC-bearing tumors has shown the presence of RET/PTC in all of the tumor cells. 22 3) Cytogenetic abnormalities in papillary thyroid carcinomas are sometimes restricted to single translocations involving 10q11.2, 27-29 the location of RET, suggesting RET/PTC could be the sole abnormality in some tumors. 4) Two transgenic mouse models of RET/PTC, driven by a thyroglobulin promoter, both develop multifocal thyroid tumors at an early age, and the tumors are histologically very similar to human papillary thyroid carcinomas, with “ground glass nuclei, grooves and inclusions”. 30,31 5) The most direct evidence that RET/PTC is sufficient to transform thyroid epithelial cells comes from gene transfer studies. When expressed in primary cultures of normal human thyroid epithelium, RET/PTC is able by itself to drive proliferation sufficiently to produce colonies of up to 10 6 cells with a characteristic fenestrated appearance by phase contrast microscopy. 32 Following on this observation, the aim of the present study was to determine whether RET/PTC is sufficient to cause the intriguing nuclear morphological alterations characteristic of papillary thyroid carcinoma.
Materials and Methods
Primary cultures of normal human thyroid cells were produced and gene transfer carried out as previously described. 32 This culture system is ∼99% epithelial as judged by cytokeratin immunostaining. 32 Briefly, 2 days after plating, cells were infected with an amphotrophic (psi-CRIP) RET/PTC-1 retroviral vector, also conferring G418 (neomycin) resistance. 32 RET/PTC-1 is a naturally occurring papillary thyroid carcinoma oncogene formed from part of a protein called H4, bearing a leucine zipper dimerization domain, fused amino-terminal to the tyrosine kinase domain of RET. Control infections were performed with an activated H-RAS-neomycin retroviral vector (psi-CRIP-DOEJ), as previously described. 32 Four days after infection, cells were passaged onto Permanox (Nunc, Naperville, IL) plastic tissue culture slides either with or without G418 selection. After one or two more weeks in culture, cells were fixed by immersion in 95% ethanol and then spayed with a solution of polyethylene glycol in alcohol (Fix-Rite, Richard-Allen, Kalamazoo, MI) and stored dry. Modified Papanicolaou staining (Richard-Allen) was performed according to standard protocols except that slides were coverslipped with glycerol (Sigma Chemical Co., St. Louis, MO) instead of xylene-based media after the last absolute alcohol wash, as the slides are plastic.
Immunohistochemistry with a polyclonal rabbit antibody directed against the tyrosine kinase domain of RET (Santa Cruz Biotechnology, catalogue item SC-167, Santa Cruz, California) was titered against control tissue culture NIH3T3 cells transfected with RET/PTC-1 (kindly provided by Dr. Sissy Jhiang) and nontransfected NIH3T3 cells. As the tyrosine kinase domain of RET is preserved in the translocation, this antibody is able to detect the rearranged version of RET in addition to proto-RET. The following protocol was used. The fixed cultures were rehydrated for 10 minutes in a 1:1 mixture of water and 95% flex alcohol (Richard-Allen), followed by three changes of TMS buffer (50 mmol/L Tris, pH 7.6, 5 mmol/L MgCl2, 150 mmol/L NaCl containing 10 μm/ml bovine serum albumin; Sigma). Blocking with 10% bovine serum plus blocking reagent from Elite Vectastain (Vector Laboratories, Burlingame, CA) diluted into TMS buffer was performed for 20 minutes at room temperature. Rabbit anti-RET antibody was diluted 1:80 into the blocking cocktail and incubated under a coverslip for 1 hour at 37°C. No antigen retrieval was used. Subsequent steps were performed with Vectastain Universal Quick Kit with the ABC method and with peroxidase-diaminobenzidine development as per the manufacturer’s instructions (Vector Laboratories catalogue items PK-8800 and SK4100) except that two 10-minute incubations with diaminobenzidine (DAB) were used. Good discrimination between RET/PTC-positive and -negative NIH3T3 cells was obtained with this protocol, and alcohol-fixed morphology was well preserved. Positive immunostaining with this protocol was observed only in thyroid cultures retrovirally infected with RET/PTC. Thyroid cell cultures not infected with RET/PTC showed no staining, indicating that C cells are either not present or are not detected. A Papanicolaou stain was performed after DAB treatment, and cells were coverslipped with glycerol.
For RAS immunostaining, a rat anti-RAS monoclonal antibody (Santa Cruz Biotechnology catalogue item SC-35) was titered against NIH3T3 cells overexpressing H-RAS (Ciras-2 cells, a gift of Jim Wright), using NIH3T3 parental cells as a negative control. The staining protocol was the same for RET with the following exceptions. Fixation did not include polyethylene glycol as this was found to diminish staining intensity. The anti-RAS antibody was diluted 1:100. A biotinylated goat anti-rat immunoglobulin (Vector Laboratories BA-9400) was used as a secondary antibody at recommended concentration. The ABC reagents from the Vector Elite System (catalogue item PK-6101) were used at recommended concentrations.
Microscopic analysis was performed with a 100× oil-immersion objective with a 1.25 numerical aperture. Criteria were established by one cytopathologist that were likely to allow discimination between papillary thyroid carcinoma nuclei and normal thyroid nuclei. An irregular nuclear contour plus areas of open chromatin were used to define a cell as papillary thyroid carcinoma-like. For nuclear contour irregularity, it was found that it was easier to look for a smooth arc in nuclear contour rather than try to quantify the degree of irregularity. An arbitrary cut off of one-fourth or greater of the nuclear perimeter in a mid-nuclear plane with a smooth round contour was used to exclude the papillary thyroid carcinoma-like phenotype. Use of the criterion of a smooth contour for one-half the nuclear perimeter was more difficult to score due to nuclear undulations or occasional shallow irregularities of normal cells. To qualify as papillary thyroid carcinoma-like, cells also had to demonstrate a 2-μm-diameter intranuclear zone devoid of hematoxylin staining texture. This measurement was performed with a micrometer. To justify these criteria, a second cytopathologist, blinded to the nature of the study, was asked to identify differences between slides of cells retrovirally infected with RET/PTC compared with RAS and then later score the morphology using the criteria above. Both cytopathologists scored all nuclei in a random scan. Nuclei that could not be scored were counted as indicated, and these included mitotic cells and obscured, degenerated, or apoptotic cells.
Results
Appearance of Nontransfected Normal Thyroid Cells in Short-Term Culture
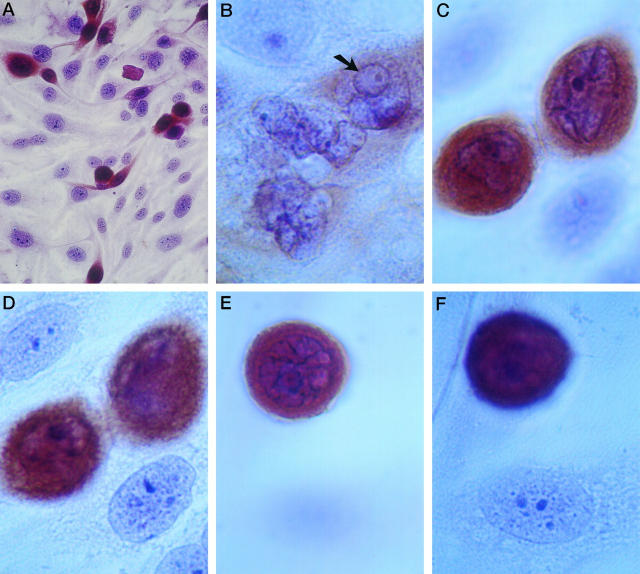
Nontransfected normal human thyroid cells in culture have nuclei that are flattened toward the substrate, with finely granular chromatin extending throughout the nucleoplasm, variable-sized nucleoli, and a smooth, round to oval nuclear contour. Figure 1, A, D, and F ▶ , shows normal thyroid epithelial cells (without brown anti-RET immunostain reaction product). The flattening of the nuclei is similar to what is seen in many other tissue culture cells and may also be seen in normal thyroid epithelium in vivo. The chromatin of cultured normal thyroid cells has a slightly finer texture on average compared with thyroid cells in vivo.
Figure 1.
Human thyroid epithelial cells expressing RET/PTC disperse and have an altered nuclear morphology. Tissue culture plates are all shown after anti-RET antibody immunostaining (brown DAB reaction product) and a Papanicolaou stain. A: RET/PTC-expressing cells disperse over the culture plate. Magnification, ×400. B: Three cells with relatively weak RET immunostaining are shown. One cell has an intranuclear cytoplasmic inclusion (arrow). The other two cells show highly irregular nuclear contours. Magnification, ×2500. C and D: The same field of view is shown at two different focal planes. The RET/PTC-expressing cells show irregular nuclear contours around most of their perimeter with nuclear grooves and areas of absent intranuclear hematoxylin staining texture. The non-immunostained cells are in a plane closer to the substrate; they show relatively round nuclear contours and a finely granular chromatin texture throughout the nucleus. Magnification, ×2500. E and F: Another field is photographed at two different focal planes to reveal RET/PTC-expressing (E) and non-expressing (F) cells. The expressing cell is traversed with numerous folds of the nuclear membrane such that less than one-fourth of the nuclear perimeter has a smooth contour. The non-expressing cells have chromatin aggregates extending throughout the nucleoplasm, whereas the RET/PTC-expressing cell has a euchromatic nucleus such that areas larger than 2 μm in diameter without hematoxylin staining texture can be identified. Magnification, ×2500.
Appearances of the RET/PTC-Transfected Cells
Cultured thyroid cells expressing RET/PTC tend to disperse among the non-expressing cells on the culture plate, as demonstrated by RET immunostaining (brown DAB reaction product) (Figure 1A) ▶ . They form occasional characteristic fenestrated colonies, previously described by low-magnification phase contrast microscopy. 32 The cytological features of these RET/PTC transfectants are different from the background cells (Figure 1, B, C, and E) ▶ . 1) The majority of the nuclei of RET/PTC-expressing cells are not flattened toward the substrate, as illustrated in Figure 1, C–F ▶ . To show the nuclei of RET/PTC-expressing cells and non-expressing cells within the same field of view, the two sets of pictures in Figure 1, C and D ▶ , and Figure 1, E and F ▶ ▶ , are required to be in two different focal planes. 2) Many RET/PTC-expressing cells have irregular, unpredictable folds of the nuclear membrane (Figure 1, B, C, and E) ▶ . 3) They tend to have open chromatin with relatively large areas showing no evident hematoxylin staining texture (Figure 1 ▶ B, C, and E). A second cytopathologist, initially blinded to the cell types and genes studied, easily discerned a difference between RET/PTC-infected and H-RAS-infected cells and described this difference as follows: 1) presence of frequent nuclear contour irregularities, 2) open chromatin, and 3) variably prominent nucleoli of RET/PTC-infected compared with RAS-infected cells.
To directly examine the relationship between RET/PTC expression and altered nuclear morphology, cells were scored for the presence of the papillary thyroid carcinoma-like morphology (defined in Materials and Methods) and RET/PTC expression. Normal human thyroid epithelial cells were scored after having been infected 10 days previously with RET/PTC, grown without G418 selection, and immunostained for RET/PTC. Cytopathologist 1 determined the proportions of papillary thyroid carcinoma-like cells and RET/PTC-immunostained cells from a random population of 2000 intact cells. The results (Table 1) ▶ show that 42% of RET/PTC-expressing cells show a papillary thyroid carcinoma-like phenotype compared with only 2.5% of non-RET/PTC-expressing cells (P ≪ 0.0001 by χ 2 analysis). A second independent cytopathologist, given the same criteria for scoring, but blinded to the cell type and gene studied, scored 500 immunostained (RET/PTC-expressing) and 500 non-immunostained cells. The second cytopathologist included all of the nuclei encountered in a random scan, even if they were non-scorable. The results (Table 1) ▶ independently show RET/PTC expression to be highly associated with a papillary thyroid carcinoma-like phenotype with 52% of the RET/PTC-expressing cells showing a papillary thyroid carcinoma-like phenotype compared with 6% of the non-expressing cells (a maximum of 9% if every non-scorable nucleus were to have had papillary thyroid carcinoma-like features; P ≪ 0.0001).
Table 1.
Association of RET/PTC Expression with an Irregular Nuclear Contour and Euchromatin
| Observer 1 | Observer 2 | |||
|---|---|---|---|---|
| RET/PTC immunostained cells | No immunostaining | 500 RET/PTC immunostained cells | 500 non-immunostained cells | |
| PTC-like phenotype | 25 | 48 | 257 | 32 |
| Not PTC-like | 34 | 1893* | 232 | 453 |
| Non-scorable | 11 | 15 |
A PTC-like phenotype was defined as an irregular nuclear contour over more than three-fourths of the nuclear perimeter and a 2-μm-diameter area of absent hematoxylin staining texture. Any nucleus that was degenerated, apoptotic, mitotic, or obscured was counted as non-scorable.
* P < 0.0001 by χ2 analysis.
The immunostaining of RET/PTC is diffuse throughout the cytoplasm with no apparent accentuation of the perinuclear or other sub-cytoplasmic domain. Rare intranuclear cytoplasmic inclusions were observed (Figure 1B) ▶ , and only in RET/PTC-infected cells.
Morphological Effects of Retroviral Gene Transfer and Transformation
Primary normal human thyroid cells transfected with H-RAS tend to grow contiguously to form a discrete colony that pushes back the surrounding normal monolayer (Figure 2A) ▶ , as previously described by phase contrast microscopy. 32 The nuclei of these cells show a slight coarsening of chromatin and are more spherical than those of nontransfected thyroid cells (Figure 2B ▶ , with brown anti-RAS immunostaining product). To quantify the difference in appearance of RAS compared with RET/PTC-infected cells, two cytopathologists separately scored plates of G418-selected human thyroid cells infected with either H-RAS or RET/PTC. A total of 1000 cells were scored for the presence or absence of the papillary thyroid carcinoma-like phenotype using the previously defined criteria. One cytopathologist was initially blinded to the nature of the study. The results, shown in Table 2 ▶ , demonstrate that the proportion of cells with a papillary thyroid carcinoma-like phenotype is ∼15% in G418-selected RET/PTC-infected cultures compared with ∼1% in the corresponding RAS-infected cultures (P ≪ 0.0001). In the case of RET/PTC, this figure is less in the G418-selected cultures than in the nonselected culture in which RET/PTC expression is assessed by immunohistochemistry (approximately 15% compared with 50%). This can be explained by the fact that the G418 resistance (neomycin) and RET/PTC portions of the construct are encoded by separate, independent cistrons. Although any cell surviving G418 selection must have been retrovirally infected and express the neomycin gene, one-half or fewer of these survivors are expected to also express the RET/PTC portion of the construct, as previously described for many other expression constructs. 33
Figure 2.
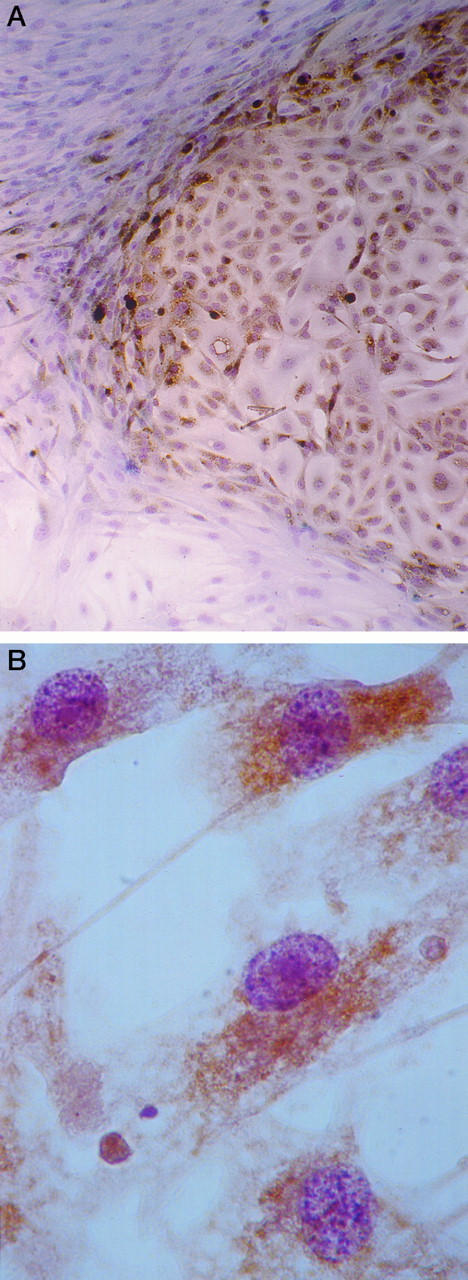
RAS expression in human thyroid cells leads to an expansive growth pattern, and nuclear features are different from those of RET/PTC-expressing cells. A: A discrete colony of H-RAS-infected human thyroid cells in cell culture is shown after anti-RAS immunostaining (brown DAB reaction product) and a Papanicolaou stain. Magnification, ×100. B: RAS-expressing human thyroid cells in cell culture show slightly coarser chromatin compared with normal thyroid cells (see, for example, the cells in Figure 1, D and F ▶ ) and round nuclear contours. Magnification, ×1000.
Table 2.
Morphological Effects of H-RAS Compared with RET/PTC*
| RET/PTC-infected | RAS-infected | |||
|---|---|---|---|---|
| Observer 1 | Observer 2 | Observer 1 | Observer 2 | |
| PTC-like phenotype | 168 | 135 | 20 | 3 |
| Not PTC-like | 778 | 785 | 970 | 990 |
| Non-scorable | 54 | 80 | 10 | 7 |
Neomycin-H-RAS and neomycin-RET/PTC retrovirally infected cells after G418 selection were counted. A PTC-like phenotype was defined as an irregular nuclear contour over three-fourths of the nuclear perimeter and a 2-μm-diameter area of absent hematoxylin staining texture.
* Probability that the frequency of the PTC-like phenotype is the same for RET/PTC- as for RAS-infected cells for either observer is < 0.0001.
At least two separate human thyroid primary cultures, transfections, and immunostaining procedures were performed, with similar results. The RET/PTC-positive immunostaining is not due to the presence of possible C cells, as no staining was observed in control cultures that were not transfected with RET/PTC. G418 does not affect the appearance of either the RAS-transfected or the RET/PTC-transfected cells.
Effects of RET/PTC on NIH3T3 Cells
The NIH3T3 cells transfected with RET/PTC and used as a control for the immunostaining do not show the same morphological changes as human thyroid epithelium; they mostly show round nuclear contours. This could be because this cell line does not express RET/PTC as strongly as the primary thyroid cells, as indicated by a relatively weaker immunohistochemical staining. Alternatively, the genetic background of these NIH3T3 cells may not permit the papillary thyroid carcinoma-like morphological phenotype to be expressed. RET/PTC-transfected NIH3T3 cells show a different growth pattern compared with nontransfected NIH3T3 cells. The transfected fibroblasts grow as either widely separated, loosely attached individual cells or as dense, loosely attached three-dimensional balls of cells. Their dispersion is reminiscent of the fenestrated appearance of RET/PTC-transfected human thyroid epithelial cells. Nontransfected NIH3T3 cells grow as a flat monolayer that spreads out evenly across the substrate.
Discussion
We show that infection of short-term cultures of normal human thyroid cells with a retrovirus vector expressing activated RET/PTC alters nuclear morphology. The alteration consists of a combination of irregular nuclear contours and development of a euchromatic change in the chromatin, similar or identical to the changes diagnostic of papillary thyroid carcinoma. The nuclear morphological changes are specific to RET/PTC and are not a consequence of gene transfer, increased proliferation, or transformation per se, as retroviral infection with a RAS construct leads to a round nuclear contour and slightly coarser chromatin. These findings add support for the hypothesis that RET/PTC is sufficient to induce papillary thyroid carcinomas in one genetic step.
Although highly significant in a population of 500 to 1000 cells, the correlation of RET/PTC expression and morphology on a cell-by-cell basis is not perfect. Some cells clearly express RET/PTC but do not show any nuclear morphological change compared with nontransfected cells. One partial explanation could be expression in a non-epithelial cell, approximately 1% of the cells in the starting culture. 32 It seems likely that fibroblasts or endothelial cells would not show the morphological changes if infected with RET/PTC as NIH3T3 cells expressing RET/PTC do not seem to show the morphological features of papillary thyroid carcinoma. Not every cell in a human papillary thyroid carcinoma necessarily shows diagnostic nuclear changes. Conversely, occasional cells that do not appear to express RET/PTC do show irregular nuclear envelope contours and open chromatin. It is possible that the level of expression of RET/PTC in some of these cells is just below the limit of detection. Also, there is a known tendency for normal thyroid cells to be able to show slight clearing of the chromatin or irregular nuclear contours without being transformed in certain conditions (eg, in Hashimoto’s thyroiditis or Graves disease). It is possible that expression of tyrosine kinases or their downstream signaling molecules can occur as a physiological event under some circumstances in normal thyroid cells, accounting for these observations.
RET/PTC activation is not found in roughly 60% of papillary thyroid carcinomas. In some of these, specific translocations involving another tyrosine kinase, TRK, may serve a precisely analogous function. 34 TRK activations are found in ∼17% of papillary thyroid carcinomas. 34 The non-rearranged versions of TRK and RET share many features. Both are normally expressed in neural-derived tissues and are not significantly expressed in thyroid follicular cells. The ligands of both TRK (nerve growth factor) and RET function in neural tissues. The rearranged version of TRK and RET (TRK/PTC and RET/PTC) share the following properties. 1) The rearrangements are frequently intrachromosomal. 7,34 2) The translocated sequences provide essential dimerization or oligomerization domains. 35 3) Both bind to the signaling intermediates SHC and GRB2 in NIH3T3 cells. 36 4) When introduced into NIH3T3 cells, both RET/PTC and TRK/PTC induce a mitogenic response, 21,37 whereas both trigger a similar differentiation response when introduced into PC12 pheochromocytoma cells. 38,39 5) Activations of TRK via translocations are virtually restricted to papillary thyroid carcinoma. 6) TRK/PTC activation appears to be an early event in the formation of some papillary thyroid carcinomas. 7) No differences in the pathological features of TRK/PTC- and RET/PTC-associated papillary thyroid carcinomas have been observed. 36 8) Both cooperate with RAS in the full transformation to tumorigenicity in a rat thyroid epithelial model. 40 In fact, the only indications that RET/PTC and TRK/PTC function differently is that RET/PTC causes a stronger loss of differentiation compared with TRK/PTC in the rat thyroid epithelial model (decreased thyroglobulin gene expression and decreased thyroid-stimulating hormone receptor gene expression), although this difference may be related to different expression levels of the transfected RET/PTC compared with TRK/PTC. 40 A prediction is that TRK/PTC should cause the same nuclear structural changes as RET/PTC. In those cases of typical papillary thyroid carcinomas in which neither RET/PTC nor TRK/PTC rearrangements are present, other tyrosine kinases or downstream signaling molecules may be predicted to mediate the change in nuclear morphology.
Our immunostaining confirms that RET/PTC is diffusely cytoplasmic. 23 There is no apparent relation of the staining to nuclear invaginations or rare inclusions. The mechanism by which RET/PTC activation could possibly lead to the change in nuclear morphology is unclear. Often, receptor tyrosine kinases act via RAS-dependent signaling pathways. However, as RAS expression leads to a different morphology, the signal for nuclear morphological changes downstream of RET/PTC does not depend entirely (if at all) on RAS. Studies of the mechanism of transformation by RET/PTC have mostly used rodent fibroblasts and assumed that the pathway to mitotic signaling is the important downstream event of RET/PTC, even though papillary thyroid carcinomas have slow growth rates. Such studies have implicated a RAS-dependent pathway, 36 a protein-kinase-C-dependent pathway, 41 and a pathway via a recently described signaling molecule called enigma. 42 However, the fibroblast system may not be adequate to analyze the function of RET/PTC as RET/PTC seems to show a different morphological effect on fibroblasts compared with thyroid epithelium, and fibroblasts in vivo have never been shown to have an activated RET/PTC. Studies in thyroid cells have shown that RET/PTC decreases in the activity of two transcription factors required for thyroid-specific gene activation, Pax-8 and TTF-1. 43
The ability of an oncogene to effect a change in large-scale chromatin organization should not be surprising. During differentiation, there are characteristic (diagnostic) changes in heterochromatin packaging patterns, and oncogenes are involved in differentiation programming. Increased chromatin coarseness and more spherical nuclear shape is seen in fibroblasts transfected with RAS, and these nuclear changes appear functionally significant. 44 We observe a mild coarsening of the chromatin in association with RAS activation in the thyroid follicular cells very similar to the changes in fibroblast nuclear morphology associated with RAS activation. An association of oncogene activation with nuclear envelope reorganization could also be anticipated. The nuclear envelope is disturbed in a wide variety of human tumors, and the types of nuclear envelope abnormalities are sometimes tumor type or pathway specific. The nuclear envelope plays an essential but incompletely understood role in replication competence. 45,46 The nuclear envelope also has a role in defining the spatial organization of nuclear pores 47 and can likely influence transcription via heterochromatinization. 48,49 It is possible, therefore, that the nuclear structural changes associated with RET/PTC expression are relevant to its function as an oncogene. Studies are underway to determine the structural basis and functional significance of RET/PTC-induced nuclear reorganization.
Footnotes
Address reprint requests to Dr. Andrew Fischer, Department of Pathology, Emory University Hospital, 1364 Clifton Road NE, Atlanta, GA 30322. E-mail afische@emory.edu.
References
- 1.LiVolsi VA: Surgical Pathology of the Thyroid, vol. 22 in series Major Problems in Pathology. Edited by Bennington JL. Philadelphia, WB Saunders, 1990
- 2.Rosai J, Carcangiu ML, Delellis RA: Tumors of the Thyroid Gland, Fascicle 5, third series. Edited by Rosai J, Sobin LH. Washington, DC, Armed Forces Institute of Pathology, 1992
- 3.Franssila KO, Ackerman LV, Brown CL, Hedinger CE: Follicular carcinoma. Semin Diagn Pathol 1985, 2:101-122 [PubMed] [Google Scholar]
- 4.Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, Wynford-Thomas D: High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989, 4:159-164 [PubMed] [Google Scholar]
- 5.Wright PA, Lemoine NR, Mayall ES, Wyllie FS, Hughes D, Williams ED, Wynford-Thomas D: Papillary and follicular thyroid carcinomas show a different pattern of ras oncogene mutation. Br J Cancer 1989, 60:576-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro M, Carlomagno F, Hay ID, Herrmann MA, Grieco M, Melillo R, Pierotti MA, Bongarzone I, Della Porta G, Berger N, Peix JL, Paulin C, Fabien N, Vecchio G, Jenkins RB, Fusco A: Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest 1992, 89:1517-1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro M, Grieco M, Melillo RM, Fusco A, Vecchio G: Molecular defects in thyroid carcinomas: role of the RET oncogene in thyroid neoplastic transformation. Eur J Endocrinol 1995, 133:513-522 [DOI] [PubMed] [Google Scholar]
- 8.Wynford-Thomas D: Origin and progression of thyroid epithelial tumours: cellular and molecular mechanisms. Hormone Res 1997, 47:145-157 [DOI] [PubMed] [Google Scholar]
- 9.Johannessen JV, Sobrinho-Simoes M: Well differentiated thyroid tumors: problems in diagnosis and understanding. Pathol Annu 1983, 18:255-285 [PubMed] [Google Scholar]
- 10.Salmon I, Kiss R, Franc B, Gasperin P, Heimann R, Pasteels JL, Verhest A: Comparison of morphonuclear features in normal, benign and neoplastic thyroid tissue by digital cell image analysis. Anal Quant Cytol Histol 1992, 14:47-54 [PubMed] [Google Scholar]
- 11.Vickery AL, Jr, Carcangiu ML, Johannessen JV, Sobrinho-Simoes M: Papillary carcinoma. Semin Diagn Pathol 1985, 2:90-100 [PubMed] [Google Scholar]
- 12.Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ: Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery 1992, 112:1139-1147 [PubMed] [Google Scholar]
- 13.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM: GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 1996, 85:1113-1124 [DOI] [PubMed] [Google Scholar]
- 14.Santoro M, Sabino N, Ishizaka Y, Ushijima T, Carlomagno F, Cerrato A, Grieco M, Battaglia C, Martelli ML, Paulin C, Fabien N, Sugimura T, Fusco A, Nagao M: Involvement of RET oncogene in human tumours: specificity of RET activation to thyroid tumours. Br J Cancer 1993, 68:460-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizaka Y, Kobayashi S, Ushijima T, Hirohashi S, Sugimura T, Nagao M: Detection of retTPC/PTC transcripts in thyroid adenomas and adenomatous goiter by an RT-PCR method. Oncogene 1991, 6:1667-1672 [PubMed] [Google Scholar]
- 16.Bounacer A, Wicker R, Caillou B, Cailleux AF, Sarasin A, Schlumberger M, Suarez HG: High prevalence of activating ret proto-oncogene rearrangements in thyroid tumors from patients who had received external radiation. Oncogene 1997, 15:1263-1273 [DOI] [PubMed] [Google Scholar]
- 17.Jhiang SM, Mazzaferri EL: The ret/PTC oncogene in papillary thyroid carcinoma. J Lab Clin Med 1994, 123:331-337 [PubMed] [Google Scholar]
- 18.Jhiang SM, Smanik PA, Mazzaferri EL: Development of a single-step duplex RT-PCR detecting different forms of ret activation and identification of the third form of in vivo ret activation in human papillary thyroid carcinoma. Cancer Lett 1994, 78:69-76 [DOI] [PubMed] [Google Scholar]
- 19.Viglietto G, Chiappetta G, Martinez-Tello FJ, Fukunaga FH, Tallini G, Rigopoulou D, Visconti R, Mastro A, Santoro M, Fusco A: RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene 1995, 11:1207-1210 [PubMed] [Google Scholar]
- 20.Tong Q, Xing S, Jhiang SM: Leucine zipper-mediated dimerization is essential for the PTC1 oncogenic activity. J Biol Chem 1997, 272:9043-9047 [DOI] [PubMed] [Google Scholar]
- 21.Durick K, Yao VJ, Borrello MG, Bongarzone I, Pierotti MA, Taylor SS: Tyrosines outside the kinase core and dimerization are required for the mitogenic activity of RET/ptc2. J Biol Chem 1995, 270:24642-24645 [DOI] [PubMed] [Google Scholar]
- 22.Klugbauer S, Demidchik EP, Lengfelder E, Rabes HM: Detection of a novel type of RET rearrangement (PTC5) in thyroid carcinomas after Chernobyl and analysis of the involved RET-fused gene RFG5. Cancer Res 1998, 58:198-203 [PubMed] [Google Scholar]
- 23.Ishizaka Y, Shima H, Sugimura T, Nagao M: Detection of phosphorylated retTPC oncogene product in cytoplasm. Oncogene 1992, 7:1441-1444 [PubMed] [Google Scholar]
- 24.Ito T, Seyama T, Iwamoto KS, Mizuno T, Tronko ND, Komissarenko IV, Cherstovoy ED, Satow Y, Takeichi N, Dohi K, Akiyama M: Activated RET oncogene in thyroid cancers of children from areas contaminated by Chernobyl accident. Lancet 1994, 344:259. [DOI] [PubMed] [Google Scholar]
- 25.Klugbauer S, Lengfelder E, Demidchik EP, Rabes HM: High prevalence of RET rearrangement in thyroid tumors of children from Belarus after the Chernobyl reactor accident. Oncogene 1995, 11:2459-2467 [PubMed] [Google Scholar]
- 26.Tallini G, Santoro M, Helie M, Carlomagno F, Salvatore G, Chiappetta G, Carcangiu ML, Fusco A: RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res 1998, 4:287-294 [PubMed] [Google Scholar]
- 27.Roque L, Clode AL, Gomes P, Rosa-Santos J, Soares J, Castedo S: Cytogenetic findings in 31 papillary thyroid carcinomas. Genes Chromosomes & Cancer 1995, 13:157-162 [DOI] [PubMed] [Google Scholar]
- 28.Sozzi G, Bongarzone I, Miozzo M, Cariani CT, Mondellini P, Calderone C, Pilotti S, Pierotti MA, Della Porta G: Cytogenetic and molecular genetic characterization of papillary thyroid carcinomas. Genes Chromosomes & Cancer 1992, 5:212-218 [DOI] [PubMed] [Google Scholar]
- 29.Sozzi G, Bongarzone I, Miozzo M, Borrello MG, Blutti MG, Pilotti S, Della Porta G, Pierotti MA: A t(10;17) translocation creates the RET/PTC2 chimeric transforming sequence in papillary thyroid carcinoma. Genes Chromosomes & Cancer 1994, 9:244-250 [DOI] [PubMed] [Google Scholar]
- 30.Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C: Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology 1996, 137:375-378 [DOI] [PubMed] [Google Scholar]
- 31.Santoro M, Chiappetta G, Cerrato A, Salvatore D, Zhang L, Manzo G, Picone A, Portella G, Santelli G, Vecchio G, Fusco A: Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene 1996, 12:1821-1826 [PubMed] [Google Scholar]
- 32.Bond JA, Wyllie FS, Rowson J, Radulescu A, Wynford-Thomas D: In vitro reconstruction of tumour initiation in a human epithelium. Oncogene 1994, 9:281-290 [PubMed] [Google Scholar]
- 33.Rees S, Coote J, Stables J, Goodson S, Harris S, Lee MG: Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques 1996, 20:102-110 [DOI] [PubMed] [Google Scholar]
- 34.Pierotti MA, Bongarzone I, Borrello MG, Mariani C, Miranda C, Sozzi G, Greco A: Rearrangements of TRK proto-oncogene in papillary thyroid carcinomas. J Endocrinol Invest 1995, 18:130-133 [DOI] [PubMed] [Google Scholar]
- 35.Greco A, Fusetti L, Miranda C, Villa R, Zanotti S, Pagliardini S, Pierotti MA: Role of the TFG N-terminus and coiled-coil domain in the transforming activity of the thyroid TRK-T3 oncogene. Oncogene 1998, 16:809-816 [DOI] [PubMed] [Google Scholar]
- 36.Borrello MG, Pelicci G, Arighi E, De Filippis L, Greco A, Bongarzone I, Rizzetti M, Pelicci PG, Pierotti MA: The oncogenic versions of the Ret and Trk tyrosine kinases bind Shc and Grb2 adaptor proteins. Oncogene 1994, 9:1661-1668 [PubMed] [Google Scholar]
- 37.Greco A, Pierotti MA, Bongarzone I, Pagliardini S, Lanzi C, Della Porta G: TRK-T1 is a novel oncogene formed by the fusion of TPR and TRK genes in human papillary thyroid carcinomas. Oncogene 1992, 7:237-242 [PubMed] [Google Scholar]
- 38.Califano D, Monaco C, de Vita G, A DA, Dathan NA, Possenti R, Vecchio G, Fusco A, Santoro M, de Franciscis V: Activated RET/PTC oncogene elicits immediate early and delayed response genes in PC12 cells. Oncogene 1995, 11:107-112 [PubMed] [Google Scholar]
- 39.Greco A, Orlandi R, Mariani C, Miranda C, Borrello MG, Cattaneo A, Pagliardini S, Pierotti MA: Expression of TRK-T1 oncogene induces differentiation of PC12 cells. Cell Growth Differ 1993, 4:539-546 [PubMed] [Google Scholar]
- 40.Santoro M, Melillo RM, Grieco M, Berlingieri MT, Vecchio G, Fusco A: The TRK and RET tyrosine kinase oncogenes cooperate with ras in the neoplastic transformation of a rat thyroid epithelial cell line. Cell Growth Differ 1993, 4:77-84 [PubMed] [Google Scholar]
- 41.Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti MG, Mondellini P, Radice MT, Pierotti MA: The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol 1996, 16:2151-2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durick K, Wu RY, Gill GN, Taylor SS: Mitogenic signaling by Ret/ptc2 requires association with enigma via a LIM domain. J Biol Chem 1996, 271:12691-12694 [DOI] [PubMed] [Google Scholar]
- 43.De Vita G, Zannini M, Cirafici AM, Melillo RM, Di Lauro R, Fusco A, Santoro M: Expression of the RET/PTC1 oncogene impairs the activity of TTF-1 and Pax-8 thyroid transcription factors. Cell Growth Differ 1998, 9:97-103 [PubMed] [Google Scholar]
- 44.Fischer AH, Chaddee DN, Wright JA, Gansler TS, Davie JR: Ras-associated nuclear structural change appears functionally significant and independent of the mitotic signaling pathway. J Cell Biochem 1998, 70:130-140 [PubMed] [Google Scholar]
- 45.Thommes P, Blow JJ: The DNA replication licensing system. Cancer Surv 1997, 29:75-90 [PubMed] [Google Scholar]
- 46.Yang L, Guan T, Gerace L: Lamin-binding fragment of LAP2 inhibits increase in nuclear volume during the cell cycle and progression into S phase. J Cell Biol 1997, 139:1077-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohno M, Fornerod M, Mattaj IW: Nucleocytoplasmic transport: the last 200 nanometers. Cell 1998, 92:327-336 [DOI] [PubMed] [Google Scholar]
- 48.Ye Q, Worman HJ: Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J Biol Chem 1996, 271:14653-14656 [DOI] [PubMed] [Google Scholar]
- 49.Ye Q, Callebaut I, Pezhman A, Courvalin JC, Worman HJ: Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J Biol Chem 1997, 272:14983-14989 [DOI] [PubMed] [Google Scholar]