Abstract
Myosin heavy chain (MHC) isoform expression was evaluated by immunohistochemistry and reverse transcription polymerase chain reaction (RT-PCR) to clarify a possible link between gastrointestinal stromal tumor (GIST) and interstitial cells of Cajal (ICCs) in the gastrointestinal (GI) tract. Using monoclonal antibodies against MHC isoforms, 18 of 27 GISTs (67%) showed immunoreactivity for non-smooth-muscle myosin or the embryonic form of MHC (SMemb), but only one tumor showed immunoreactivity for smooth muscle cell (SMC)-specific isoforms (SM1 and SM2). Co-expression of KIT or CD34, which is also expressed in GIST and ICCs, was demonstrated in 18 (100%) and 16 SMemb-positive tumors (89%), respectively. Otherwise, the expression of SMemb in GIST was not correlated with the patient’s age or sex, tumor size, histological grade of GIST, or expression of mesenchymal cell markers, such as α-smooth muscle actin (α-SMA) or S100 protein. By double-fluorescence immunostaining of the tunica muscularis of the GI tract wall, co-expression of KIT, CD34, and SMemb was demonstrated in ICCs, which were negative for SM1 and SM2. RT-PCR analysis confirmed that GIST expressed SMemb-mRNA, which lacked neuronal cell-specific inserts of 30 bp. These facts further strengthen the current hypothesis that GIST is a tumor of ICCs.
Gastrointestinal stromal tumor (GIST) is the most frequent non-epithelial neoplasm in the stomach and intestine. 1-5 The unifying but noncommittal term GIST has been introduced to avoid the confusion and controversy regarding the origin of the tumor. In the past, GIST was simply believed to be smooth muscle in origin based on histological features. Subsequent immunohistochemical and ultrastructural studies have shown that the tumors consist of mesenchymal cells with or without differentiation to smooth muscle cell (SMC), neuronal cell, or both, 1-5 thus casting doubt on the uniformity of GIST. However, the possibility has recently been raised that GIST is not a catalog index encompassing heterologous tumors, but rather is a neoplasm that exhibits differentiation to a specific type of cell in the gastrointestinal (GI) tract. Neoplastic cells of most GISTs simultaneously express KIT and CD34 antigen, a proto-oncogenic receptor tyrosine kinase and a hematopoietic progenitor cell antigen, respectively, both of which are expressed in hematopoietic stem cells. 6,7 In normal GI tracts, the interstitial cells of Cajal (ICCs), that were described by Cajal in 1893 as primitive neurons in autonomically innervated organs, 8,9 are immunoreactive for both antigens. Gain-of-function mutations of c-kit have been demonstrated in five of six GISTs. These facts are the basis for the current hypothesis that GIST is a tumor of ICCs, or at least consists of neoplastic cells capable of differentiating to ICCs. 6,7
In an attempt to characterize neoplastic cells of GIST, in the present study we evaluated the expression profile of myosin heavy chain (MHC) isoforms SM1, SM2, and the embryonic form (SMemb). SM1 and SM2 are alternatively spliced products of a single gene. 10,11 Both are specific to SMCs but are regulated differently in fetal development. SM1 is expressed in SMCs throughout the early developmental stage to the mature stage, whereas SM2 is expressed only after birth. 11,12 SMemb is referred to as the nonmuscle MHC isoform and is a product of a different gene. In addition to its abundance in the brain, SMemb is expressed together with SM1 in SMCs undergoing growth and/or cell division, such as embryonic SMCs of fetal aorta and proliferating SMCs in arteriosclerotic neointima. 12,13 In the present study, we demonstrated that the profile of MHC isoforms in neoplastic cells of GIST was different from that in both mature and developing SMCs, but the same as that in ICCs, thus providing further evidence that GIST is a tumor of interstitial cells of Cajal (TICC).
Materials and Methods
GIST and Mesenchymal Tumors
Twenty-seven GISTs were surgically resected from 26 patients (age, 33 to 80 years; 10 male and 16 female). These tumors consisted of 24 primary tumors of 23 patients and 3 metastatic tumors of 3 patients. These tumors were examined in our previous study, and the clinicopathological details have been reported. 14 The sites of the primary tumors were the stomach (n = 18), duodenum (n = 2), jejunum (n = 1), ileum (n = 1), and colon (n = 2). The three metastatic tumors, for which primary tumors were not available, were derived from GISTs of the stomach, duodenum, and rectum, respectively. Formalin-fixed and paraffin-embedded specimens were used for histopathological and immunohistochemical studies. As a control, nine leiomyomas (three of the uterus and six of the esophagus), eight leiomyosarcomas of the uterus, and three Schwannomas of the retroperitoneum and mediastinum were similarly examined. All of the GISTs were histologically classified as high or low risk according to the criteria of Franquemont with some modifications as have been previously reported. 14
Non-Neoplastic Tissue
For the immunohistochemical study of ICCs, non-neoplastic tissues of the human stomach, small intestine, and colon, which were surgically resected, were fixed in acetone at −20°C overnight and then rinsed in methyl benzoate and xylene and embedded in paraffin (AMeX method), 15 as long cytoplasmic processes of ICCs were lost by routine formalin fixation. Fifteen-micron-thick sections were used for the visualization of long and slender dendrites of ICCs.
Immunohistochemical Study
Immunohistochemical evaluation was performed using the avidin-biotin-peroxidase complex (ABC) method in 3-μm-thick sections of formalin-fixed and paraffin-embedded specimens of GISTs and other mesenchymal tumors. Monoclonal antibodies against MHC isoforms SM1, SM2 and SMemb were purchased from YAMASA (Tokyo, Japan) and used at a working dilution of 1:6000. A polyclonal antibody for KIT was obtained from MBL (Nagoya, Japan), and a monoclonal antibody for CD34 was obtained from Becton Dickinson (Mountain View, CA). The working dilution was 1:100 and 1:20, respectively. Cellular differentiation in GISTs was partially characterized in a previous study 13 using the following antibodies: α-SMA (DAKO, Kyoto, Japan; monoclonal, working dilution, 1:500) as a marker for SMCs and S100 protein (DAKO; polyclonal, 1:1000) as a marker for neuronal cells. Ki-67 (MBL; monoclonal, 1:100) was used to assess the proportion of proliferating cells, and a Ki-67 labeling ratio was estimated as reported previously. To recover the antigenicity of Ki-67, SM1, SM2, and SMemb, formalin-fixed sections were pretreated in a microwave oven before incubation with the primary antibody.
Fluorescence Double Immunolabeling
Fluorescence double immunostaining was performed using combinations of rabbit polyclonal anti-KIT antibody with other mouse monoclonal antibodies (S100β (IBL, Fujioka, Japan; monoclonal, 1:100), CD34, SM1, SM2, and SMemb). After incubation with the combination of primary antibodies for 1 hour at room temperature, the sections were thoroughly washed with phosphate-buffered saline. They were then incubated with tetramethylrhodamine isomer R (TRITC)-conjugated swine anti-rabbit IgG (DAKO; 1:40) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (American Qualex, San Clemente, CA; 1:40) for 30 minutes at room temperature. The sections were examined with a confocal laser scanning microscope (Leica TCS NT). The excitation wavelength was 568 nm for TRITC and 488 nm for FITC.
Transcriptional Analysis of SMemb
Transcriptional expression of SMemb was analyzed by reverse transcriptase polymerase chain reaction (RT-PCR) analysis using the tumor RNA of GISTs (n = 3), leiomyomas of the uterus (n = 3), and Schwannomas (n = 3, with 2 arising from retroperitoneum and 1 from mediastinum), as well as the adult brain, which is known to contain a large amount of SMemb. All three of the tumor tissues of GISTs showed immunoreactivity for SMemb. All of the tissues had been taken immediately after resection, frozen in dry ice/hexane, and stored at −80°C until use. Total RNA was prepared from frozen tissues with TRIZOL Reagent (GIBCO BRL, Rockville, MD) according to the manufacturer’s recommendations. Two micrograms of total RNA from each sample was reverse-transcribed to cDNA using a first-strand cDNA synthesis kit (Pharmacia Biotech, Tokyo, Japan). The resulting cDNA was then used for PCR amplification with the upstream primers (5′-AGGAAGAAAGGACCATAATATTCC-3′) and the downstream primers (5′-CCTGTAGTTATTAAATCCTTCAAG-3′). The primers were set up to amplify the fragment of SMemb mRNA encompassing the N30 exon, a 30-nucleotide insert in a splicing variant, which has been reported to be specific for the brain, retina, and retinoblastoma cell line. 16-18 After acrylamide gel electrophoresis, the gel was stained with ethidium bromide. The amplified fragments were excised from the gels and used to determine the nucleotide sequences of the amplified fragments with an ABI PRISM d-rhodamine terminator cycle sequencing ready reaction kit (Applied Biosystem, Chiba, Japan) and an ABI PRISM 377 DNA sequencer.
Statistical Analysis
Fisher’s exact probability test, the Kruskal-Wallis rank test, and Student’s t-test were used for the statistical analysis.
Results
The results of immunohistochemistry are summarized in Table 1 ▶ .
Table 1.
MHC Isoforms and Other Markers in GISTs and Other Mesenchymal Tumors
| MHC isoform | KIT | CD34 | α-SMA | S-100 | |||
|---|---|---|---|---|---|---|---|
| SM1 | SM2 | SMemb | |||||
| GIST | 0 /27 | 1 /27 | 18 /27 | 23 /27 | 22 /27 | 10 /27 | 1 /27 |
| Leiomyoma | 9 /9 | 9 /9 | 0 /9 | 0 /9 | 0 /9 | 9 /9 | 0 /9 |
| Leiomyosarcoma | 5 /8 | 5 /8 | 2 /8* | 0 /8 | 0 /8 | 4 /8 | 0 /8 |
| Schwannoma | 0 /3 | 0 /3 | 0 /3† | 0 /3 | 0 /3‡ | 0 /3 | 3 /3 |
*Only focal and weak positive staining in tumor cells.
†A few SMC of the blood vessel and fibroblast-like cells in capsule were positive, but tumor cells were completely negative.
‡In one case of Schwannoma, CD34-positive fibroblast-like cells were observed in an Antoni B area.
SMemb expression in GIST
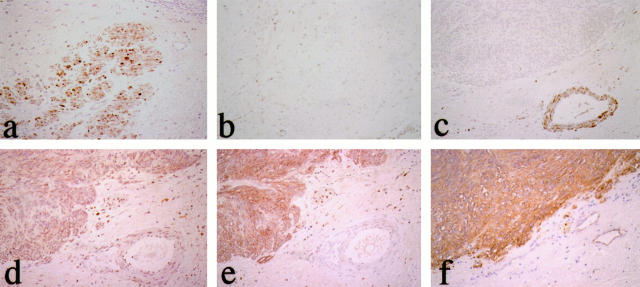
Immunohistochemically, SM1 and SM2 were definitely present in SMCs of tunica muscularis (Figure 1a) ▶ but completely absent in GISTs (Figure 1c) ▶ , except for one GIST in which several neoplastic cells showed weak positive staining. Both isoforms were present in all of the leiomyomas and in five of eight leiomyosarcomas but not in any of the Schwannomas. On the other hand, although SMemb immunoreactivity was completely negative in SMCs of the tunica muscularis (Figure 1b) ▶ , SMemb immunoreactivity was observed in neoplastic cells in 18 of 27 GISTs (67%; Figure 1d ▶ ). SMemb immunoreactivity was not demonstrated in tumor cells of leiomyomas or Schwannomas except for the SMCs of small vessels in the capsule of one of the Schwannomas. In two of the eight leiomyosarcomas, several neoplastic cells were weakly positive for SMemb, but these neoplastic cells were also immunoreactive for SM1 and SM2.
Figure 1.
Immunohistochemical evaluation of myosin heavy chain isoforms in GIST. Smooth muscle cells (SMCs) of muscular layer and blood vessels of the stomach are positive for SM1 and SM2 (a) but negative for SMemb (b). The neoplastic cells of GIST are completely negative for SM1 and SM2 (c). On the other hand, the neoplastic cells show diffusely positive staining for SMemb (d), KIT (e), and CD34 (f). ABC immunostaining; magnification, ×30.
As reported by other investigators, 6,7 most GISTs showed immunoreactivity for both KIT and CD34. All of the neoplastic cells exhibited KIT immunoreactivity in 23 of 27 GISTs (85%) (Figure 1e) ▶ but in none of the other mesenchymal tumors. Twenty-two of twenty-seven GISTs (81%) were positive for CD34 (Figure 1f) ▶ , whereas none of the leiomyomas or leiomyosarcomas showed positivity. In one case of Schwannoma, which contained an Antoni B area, many CD34-positive fibroblast-like cells were observed within the tumor nodule, as has been described previously. 19 However, neoplastic cells, which possessed plump oval nuclei, were completely negative. Thus, 18 SMemb(+) GISTs consisted of 16 KIT(+)CD34(+) and two KIT(+) CD34(−) tumors. On the other hand, nine SMemb(−) tumors consisted of four KIT(+)CD34(+), one each KIT(+)CD34(−) and KIT(−)CD34(+), and three KIT(−)CD34(−) tumors.
α-SMA immunoreactivity was observed in 10 of 27 GISTs (37%), in contrast to 9 of 9 leiomyomas and 4 of 8 leiomyosarcomas. The α-SMA immunoreactivity in GISTs was weak compared with that in leiomyomatous tumors and was confined in a focal area. Only one case of GIST showed positivity for S-100 protein.
As for the correlation between SMemb expression and pathological factors or cellular differentiation in GIST (Table 2) ▶ , SMemb expression was not correlated with sex, age, size, histological grade, or the Ki-67 labeling ratio. There was an intimate correlation between the expression of SMemb and KIT (P < 0.05), whereas there was no significant correlation between SMemb and α-SMA, CD34, or S100 protein. The expressions of KIT and CD34 were also closely correlated (P < 0.05).
Table 2.
Comparison of Clinicopathological and Immunohistochemical Results between SMemb-Positive and -Negative GISTs
| SMemb | Statistical significance | ||
|---|---|---|---|
| Positive (n = 18; 17 cases) | Negative (n = 9; 9 cases) | ||
| Sex (male/female) | 4/13 | 6/3 | NS |
| Pathological grde (high risk/low risk) | 6/12 | 6/3 | NS |
| Primary site (stomach/intestine/colon) | 15/2/1/ | 4/3/2 | NS* |
| Maximum size of the primary site (cm) | 9.1 ± 6.8 (n = 15) | 8.1 ± 5.3 (n = 9) | NS |
| Immunohistochemistry (n = 27) | |||
| α-SMA | 8/18 (44%) | 2/9 (22%) | NS |
| S100 | 1/18 (6%) | 0/9 (0%) | NS |
| CD34 | 16/18 (89%) | 5/9 (56%) | NS |
| KIT | 18/18 (100%) | 5/9 (56%) | P < 0.05 |
| Ki-67 LI (%) | 6.2 ± 5.6 | 8.4 ± 7.7 | NS |
LI (%), labeling index of Ki-67-positive tumor cells; NS, not statistically significant.
*Kruskal-Wallis rank test.
SMemb Expression in ICCs
To identify the cells corresponding to ICCs, we used KIT immunohistochemistry because of the feasibility of a polyclonal antibody. As KIT-immunoreactive cells do not always represent ICCs, the immunoreactive interstitial cells or the cells with similar morphology, which were apparently different from mast cells, were described as ICC-like cells in the present study.
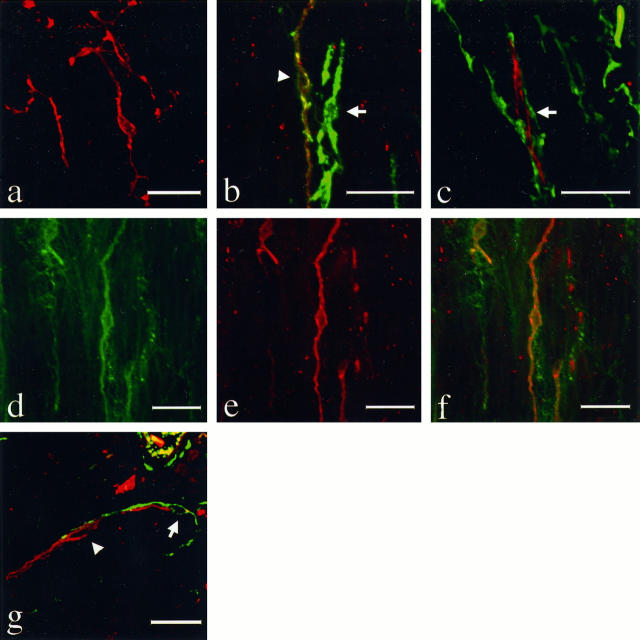
In the stomach and colon, KIT-immunoreactive ICC-like cells were distributed widely in both inner circular and outer longitudinal muscle layers of the tunica muscularis. The immunoreactive cells were fusiform or stellate in shape with long cytoplasmic processes, and formed a complex cell network (Figure 2a) ▶ . By double immunostaining, most of the KIT-immunoreactive cells were also positive for CD34, but some fusiform and stellate ICC-like cells were positive for CD34 and negative for KIT (Figure 2b ▶ , green fluorescence), and some KIT-positive ICC-like cells were negative for CD34 (Figure 2c ▶ , red). There seemed to be no difference in the morphology or location among these KIT(+)CD34(+), KIT(+)CD34(−), and KIT(−)CD34(+) ICC-like cells in the tunica muscularis. KIT-immunoreactive ICC-like cells (Figure 2e ▶ , red) were definitely positive for SMemb (Figure 2d ▶ , green; Figure 2f ▶ , yellow). These KIT-immunoreactive cells were completely negative for S100-β, SM1, and SM2.
Figure 2.
Fluorescence double immunolabeling of the interstitial cells of Cajal (ICCs) of the stomach (a to f) and small intestine (g). Immunohistochemistry was applied to 15-μm-thick sections, which had been treated by the AMeX method, using a combination of polyclonal anti-KIT antibody with monoclonal antibodies to other substances. TRITC-conjugated swine anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG were used for visualization of the immunoreaction, respectively. The sections were examined with a confocal laser scanning microscope with an excitation wavelength of 568 nm for TRITC (red) and 488 nm for FITC (green). KIT-positive ICCs (red) in the muscular layer form a complex cell-network (a, magnification, ×625). KIT-immunoreactive cells are in most cases positive with CD34 (b, left, arrowhead, magnification, ×800), but there is also a KIT-negative, CD34-positive ICCs-like cell (b, right, arrow) and KIT-positive and CD34-negative ICCs-like cells (c, arrow, magnification, ×800). On the other hand, SMemb (d) and KIT (e) immunoreactivities are observed in nearly all of the same cells (f, magnification, ×575). In the muscular layer of small intestine, most of the KIT-positive cells were negative for CD34 (g, arrowhead) but were present in the vicinity of KIT(−) CD34(+) ICCs-like cells (g, arrow, magnification, ×600). The latter type of interstitial cells were more frequent and distributed more widely in the tunica muscularis and subserosa. Bars, 20 μm.
On the other hand, the immunophenotypes and location of ICC-like cells in the small intestine were different from those in the stomach and colon. In the wall of the small intestine, many KIT-positive cells were located between the circular muscle and longitudinal muscle layer, whereas only a few KIT-positive cells were observed around the muscle bundles. Almost all of these ICC-like cells were negative for CD34 and weakly positive for SMemb. On the other hand, CD34(+)KIT(−) ICC-like cells were much more frequent, and distributed widely in the tunica muscularis and subserosa (Figure 2g) ▶ .
As an internal control for immunohistochemistry, the SMCs of the tunica muscularis were completely negative for KIT, SMemb, CD34, and S-100β protein but definitely positive for SM1 and SM2. Ganglion cells in the Auerbach plexus were negative for these antibodies.
Transcriptional Analysis of SMemb
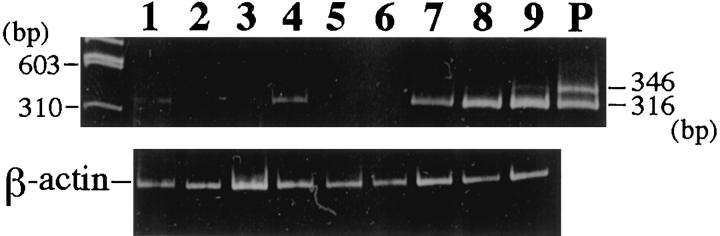
By RT-PCR analysis of SMemb mRNA, two distinct fragments were amplified in human adult brain tissue, which corresponded to transcripts with (346 bp) or without the N30 insert (316 bp). The DNA sequence of the excised fragments confirmed the specificity of the amplification. On the other hand, only a single fragment of 316 bp, corresponding to SMemb devoid of the N30 insert, was amplified in three of three GISTs. Similarly, a single fragment of 316 bp was barely amplified in each of three leiomyomas and Schwannomas (Figure 3) ▶ . With regard to immunohistochemistry, these faint fragments might correspond to the SMemb in small vessels feeding the tumors.
Figure 3.
RT-PCR analysis of SMemb in GIST. Ethidium bromide staining of acrylamide gel electrophoresis. The 346-bp fragment includes N30 whereas the 316-bp fragment does not. Both fragments are amplified in human brain mRNA (P), but a single 316-bp fragment is observed in all three GISTs, which expressed SMemb immunohistochemically (lanes 7 to 9). Similarly, a single fragment devoid of N30 is barely amplified in each of the three leiomyomas (lanes 1 to 3) and Schwannomas (lanes 4 to 6), respectively.
Discussion
In the present study, the embryonic form of MHC (SMemb), but not SM1 or SM2, was present in the neoplastic cells of GISTs by immunohistochemistry and RT-PCR analysis. As SMemb expression was not correlated with any clinicopathological factors, especially pathological grade, its expression is likely to reflect the characteristics of the normal counterpart of neoplastic cells of GISTs. The mature SMC expresses both SM1 and SM2, which are associated with its contractile phenotype. The growing SMCs of blood vessels, such as in fetal development and in reaction to tissue injury, exhibit SM1 and SMemb, but not SM2. 11-13 Thus, the profile of MHC isoforms in GISTs is different from those in both growing and mature SMCs. Moreover, there was no correlation between the expression of SMemb and α-SMA, which is another marker protein for smooth muscle differentiation. These immunohistochemical findings were apparently different from those in leiomyoma and leiomyosarcoma, confirming that GIST is distinct from a genuine leiomyomatous neoplasm. Although SMemb mRNA in neuronal cells includes a specific 30-nucleotide insert by alternative splicing, 17,18 RT-PCR analysis of GIST mRNA demonstrated amplification of only a single fragment of SMemb transcript that lacked this insert. Therefore, if SMemb expression in the neoplastic cells of GISTs reflects the characteristics of a normal counterpart, the candidate should be cells other than SMCs and neuronal cells.
Based on the current hypothesis that GIST may be a tumor of ICCs, 6,7 we investigated SMemb expression in ICCs. Although Cajal originally considered the cells as neuronal cells, recent reports suggest that ICCs may be derived from a mesenchymal cell; ICCs can be induced in fetal GI tract by the expression of Sonic hedgehog (Shh). 20 Transcripts of both c-kit and smooth muscle myosin heavy chain genes were demonstrated in mesenchymal progenitor cells of the GI tract in the mouse embryo. 21 In the present study, KIT-positive ICC-like cells in the muscular layer of the stomach and the colon were definitely positive for SMemb by fluorescence double immunolabeling. Moreover, most of the double-positive cells also showed immunoreactivity for CD34 antigen. These findings parallel those in GIST, providing additional evidence that GIST is a tumor of ICCs (TICC) in the GI tract. Two leiomyosarcomas showed SMemb, but neoplastic cells were also immunoreactive for SM1 and SM2, suggesting that the expression of SMemb may be caused by dedifferentiation or dysregulation of MHC isoforms in these highly malignant leiomyomatous tumors.
The exact function of SMemb is still under study, but it has been reported that SMemb is expressed in large amounts in human brain 17,18 and is considered to play an important role in axon formation and the direction of outgrowth in neurons, by generating tension between growth cones or bundling of actin filaments. 22 On the other hand, ICCs have long cytoplasmic processes, like axons, and form a complex network in lamina muscularis. 23 Moreover, ICCs show an impulse conductive function, and slow-wave peristalsis is blocked in W/Wv mouse or Ws/Ws rat, in which ICCs are absent because of a loss-of-function mutation of the c-kit gene. 24,25 Thus, based on the correspondence of morphology and function in the two types of cells, SMemb may play a role in the formation and preservation of dendrites and the cell network of ICCs, although the neuron-specific N30 insert variant of SMemb was not present in GISTs.
It is worth noting that KIT(+)SMemb(+) ICC-like cells in the small intestine were negative for CD34. As most of the corresponding cells in the stomach and colon were immunoreactive for CD34, the finding may indicate that ICCs in the human GI tract may be heterogeneous with regard to phenotype. 24,27 It is possible that this variable phenotype is linked to the variable function of ICCs as other than the pacemaker of the GI tract. In this context, the term gastrointestinal pacemaker cell tumor (GIPACT), which was recently proposed by Kindblom et al as a substitute for GIST, 7 does not seem to be appropriate.
In conclusion, the concomitant expression of SMemb with KIT and CD34 was observed in both GISTs and ICCs. The findings in the present study further strengthen the current hypothesis that most GISTs are tumors of ICCs (TICCs) in the GI tract, although the functional significance of SMemb in ICCs and its related tumor, TICC, requires further clarification.
Acknowledgments
We thank Ms. M. Kikuchi for her technical assistance and Mr. T. Sakurai and Ms. K. Hidano for their photographic work.
Footnotes
Address reprint requests to Dr. Masashi Fukayama, 3311–1 Yakushiji, Minamikawachi-machi, Kawachi-gun, Tochigi 329-0431, Japan. E-mail: fukayama@jichi.ac.jp.
Supported by a Grant-in Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1.Ueyama T, Guo KJ, Hashimoto H, Daimaru Y, Enjoji M: A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer 1992, 69:947-955 [DOI] [PubMed] [Google Scholar]
- 2.Franquemont DW, Frierson HF, Jr: Muscle differentiation and clinicopathological features of gastrointestinal stromal tumors. Am J Surg Pathol 1992, 16:947-954 [DOI] [PubMed] [Google Scholar]
- 3.Ma CK, Amin MB, Kintanar E, Linden MD, Zarbo RJ: Immunohistologic characterization of gastrointestinal stromal tumors: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol 1993, 6:139-144 [PubMed] [Google Scholar]
- 4.Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM: Gastrointestinal autonomic nerve tumors. Am J Surg Pathol 1993, 17:887-897 [DOI] [PubMed] [Google Scholar]
- 5.Vrettou E, Karkavelas G, Christoforidou B, Meditskou S, Papadimitriou CS: Immunohistochemical phenotyping and PCNA detection in gastrointestinal stromal tumors. Anticancer Res 1995, 15:943-950 [PubMed] [Google Scholar]
- 6.Hirota S, Isozaki K, Yasuhiro M, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 7.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM: Gastrointestinal pacemaker cell tumor (GIPACT) Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998, 152:1259-1269 [PMC free article] [PubMed] [Google Scholar]
- 8.Cajal SR: Sur les ganglions et plexus nerveux de l’intesin. Compt Rend Soc Biol Paris 1893, 45:217-223 [Google Scholar]
- 9.Taxi J: Contribution a l’etude des connexions des neurones moteurs du systeme nervoux autonome. Ann Sci Nat Zool 1965, VII:413-474 [Google Scholar]
- 10.Nagai R, Kuro-o M, Babij P, Periasamy M: Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J Biol Chem 1989, 264:9734-9737 [PubMed] [Google Scholar]
- 11.Kuro-o M, Nagai R, Tsuchimochi H, Katoh H, Yazaki Y, Ohkubo A, Takaku F: Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem 1989, 264:18272-18275 [PubMed] [Google Scholar]
- 12.Aikawa M, Sivam PN, Kuro-o M, Kimura M, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, Nagai R: Human smooth muscle myosin heavy chain isoforms as a molecular marker s for vascular development and atherosclerosis. Circ Res 1993, 73:1000-1013 [DOI] [PubMed] [Google Scholar]
- 13.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F: cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and atherosclerosis. J Biol Chem 1991, 266:3768-3773 [PubMed] [Google Scholar]
- 14.Sakurai S, Fukayama M, Kaizaki Y, Saito K, Kanazawa K, Kitamura M, Iwasaki Y, Hishima T, Hayashi Y, Koike M: Telomerase activity in gastrointestinal stromal tumor (GIST). Cancer 1998, 83:2060-2066 [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y: The AMeX method: a simplified technique of tissue processing and paraffin-embedding with improved preservation of antigens for immunostaining. Am J Pathol 1986, 125:431-435 [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Kawamoto S, Adelstein R: Evidence for inserted sequences in the head region of nonmuscle myosin specific to the nervous system. J Biol Chem 1992, 267:17864-17871 [PubMed] [Google Scholar]
- 17.Itoh K, Adelstein R: Neuronal cell expression of inserted isoforms of vertebrate nonmuscle myosin heavy chain II-B. J Biol Chem 1995, 270:14533-14540 [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto S: Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J Biol Chem 1996, 271:17613-17616 [PubMed] [Google Scholar]
- 19.Weiss SW, Nickoloff BJ: CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol 1993, 17:1039-1045 [DOI] [PubMed] [Google Scholar]
- 20.Apelqvist A, Ahlgren U, Edlund H: Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol 1997, 7:801-804 [DOI] [PubMed] [Google Scholar]
- 21.Kluppel M, Huizinga JD, Malysz J, Bernstein A: Developmental origin and Kit-dependent development of interstitial cells of Cajal in mammalian small intestine. Dev Dyn 1998, 211:60-71 [DOI] [PubMed] [Google Scholar]
- 22.Ward SM, Burns AJ, Torihashi S, Sanders KM: Mutations of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 1994, 480:91-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochlin MW, Itoh K, Adelstein RS, Bridgman PC: Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci 1995, 108:3661-3670 [DOI] [PubMed] [Google Scholar]
- 24.Komuro T, Tokui K, Zhou DS: Identification of the interstitial cells of Cajal. Histol Histopathol 1996, 11:769-786 [PubMed] [Google Scholar]
- 25.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A: W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373:347-349 [DOI] [PubMed] [Google Scholar]
- 26.Isozaki K, Hirota S, Nakama A, Miyagawa J, Shinomura Y, Xu Z, Nomura S, Kitamura Y: Disturbed intestinal movement, bile reflux to the stomach, and deficiency of c-kit-expressing cells in Ws/Ws mutant rats. Gastroenterology 1995, 109:456-464 [DOI] [PubMed] [Google Scholar]
- 27.Rumessen JJ, Thunberg L: Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Gastroenterology 1996, 216:82-94 [DOI] [PubMed] [Google Scholar]