Abstract
We have generated transgenic mice expressing the shortest human tau protein, the microtubule-associated protein that composes paired helical filaments in Alzheimer’s disease. Transgenic tau transcripts and proteins were strongly expressed in neurons in the developing and adult brain. In contrast to the endogenous tau that progressively disappeared from neuronal cell bodies during development, the human transgenic tau remained abundant in cell bodies and dendrites of a subset of neurons in the adult. This somatodendritic transgenic tau was immunoreactive with antibodies to tau phosphorylated on Thr181 and Thr231 and with the conformation-dependent Alz50 antibody. A few astrocytes expressing the transgenic tau were strongly immunoreactive with antibodies to additional tau phosphorylation sites, ie, at Ser262/356 and Ser396/404. All of these phosphorylation sites have been identified in paired helical filaments-tau proteins. In electron microscopy, the transgenic tau was detected into microtubules in axons and in dendrites but not in cell bodies. Neurofibrillary tangles were not detected in transgenic animals examined up to the age of 19 months. These results indicate that transgenic manipulation of tau expression and intracellular targeting is sufficient per se to affect tau compartmentalization, phosphorylation, and conformation partly as it is observed at the pretangle stage in Alzheimer’s disease.
Neuronal microtubules play an important role during the extension of growth cones, in the maintenance of neuronal shape, and in fast axoplasmic transport in neuronal processes. They are composed of dimers of tubulin and of microtubule-associated proteins (MAPs). Tau protein is a MAP initially identified by its ability to drive microtubule (MT) assembly in vitro. 1 Six isoforms of tau proteins have been identified in the adult human brain, all of which are generated from one gene by alternative splicing. These isoforms differ by the presence or absence of two types of inserts in their N-terminal domain and three or four nonhomologous repeats in their half-carboxyl domain. The pattern of expression of tau isoforms is developmentally regulated. A single isoform, which has no N-terminal insert and only three nonhomologous repeats, is expressed in the embryonic brain. Tau proteins bind to tubulin via their nonhomologous repeats and adjacent sequences. This binding favors MT nucleation, stabilizes MT against depolymerizing agents, induces bundling of MT, and modulates MT dynamic instability. 2 The ability of tau to promote tubulin assembly and to stabilize MT depends on its degree of phosphorylation. 3 Highly phosphorylated tau protein stabilizes and interacts less efficiently with MT in vitro. 4,5
In the developing brain, tau is present in all neuronal compartments but is concentrated in axons of mature neurons. 6,7 This axonal targeting implies mechanisms such as selective targeting of tau mRNAs at the basis of the axon, 8 locally regulated microtubule binding, 9 and selective stabilization of tau in axons. 10 Interestingly, a cis-acting signal present in the 3′untranslated tau mRNA region has been implied in tau mRNA targeting. 11 Tau has also been identified in non-neuronal cells, eg, in oligodendrocytes and in astrocytes. 12,13
Interest for the study of tau has been fostered by its involvement in the pathogenesis of cellular lesions characteristic of several human neurodegenerative conditions. 2,14 For instance, tau is the main component of abnormal filaments called paired helical filaments (PHF). 15-18 Bundles of PHF form the neurofibrillary tangles that accumulate in neurons in Alzheimer’s disease and represent a hallmark neuropathological lesion of this disease. A major post-translational modification of the tau species composing PHF (PHF-tau proteins) is a high phosphorylation state. 19 PHF-tau proteins are inefficient in triggering MT assembly but recover this ability after dephosphorylation, and it is believed that the high degree of phosphorylation of PHF-tau is a critical event linked to microtubule disorganization and generation of neurofibrillary lesions. 20 Neurofibrillary tangles or other tau positive inclusions are also observed in the brains of many patients with familial frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17). Pathogenic mutations in the tau gene have been recently discovered in FTDP-17 patients. 21-23 Some of these mutations change the relative ratio of tau isoforms or could affect the binding of tau to microtubule and microtubule stabilization.
The formation of neurofibrillary tangles is preceded by a “pretangle” stage characterized by a somatodendritic accumulation of PHF-tau proteins. 24,25 This initial accumulation might be related to changes in the mechanisms responsible for the compartmentalization of tau in mature neurons. To investigate this possibility, we have generated transgenic mice harboring a transgene encoding the human shortest (fetal-type) tau isoform but lacking the nucleotide sequence present in the 3′untranslated tau mRNA region and implied in tau mRNA targeting. 11 We report here that the expression of this transgene leads to marked changes in tau compartmentalization and phosphorylation, mimicking partly the modifications observed at the pretangle stage in Alzheimer’s disease.
Materials and Methods
Generation and Screening of Transgenic Mice
The full-length cDNA encoding fetal human tau was kindly provided by K. Kosik (Boston, MA). This cDNA encodes the three repeat human tau isoform without N-terminal insert. 26 The cDNA sequence was amplified by polymerase chain reaction (PCR) using a sense primer containing a sequence specifically recognized by EcoRV (5′-GGGATATCGATCATATGGCTGAGCCCCGCCAGGAG-3′) and an antisense primer containing a sequence specifically recognized by SalI (5′-GTCGACGTCGACTCACAAACCCTGCTTGGCCAGGGAGGC-3′).The PCR amplification generated a PCR product with an EcoRV site followed by the initiation ATG codon at the 5′ end, and a SalI site preceded by the stop codon at the 3′ end, corresponding to the coding sequence of the tau cDNA without 5′- and 3′- untranslated sequences. The PCR product was digested by EcoRV and SalI and cloned in the pHMG vector digested by the same restriction enzymes. The structure of the hybrid gene is shown in Figure 1 ▶ . The transgene was excised by digestion with NotI, to remove all of the vector sequences and purified on an agarose gel electrophoresis according to standard procedures. 27 The linearized DNA, diluted to a final concentration of 2 ng/μl in 10 mmol/L Tris-HCl (pH 7.4), 0.1 mmol/L EDTA, was injected in one of the two pronuclei of fertilized mouse embryos (C57 black6/J × CBA-F2 hybrids, IFFA CREDO). The surviving embryos were subsequently transplanted into the oviduct of pseudopregnant foster mothers. Mice harboring the transgene were detected by Southern blot analysis of DNA prepared from tail samples. A 1200-bp PstI-Sal I fragment from the intron 1 of the pHMG plasmid randomly labeled with (α32P)-dCTP served as a probe for the detection of the transgene and as internal control. The Southern blot analysis did not show any major rearrangement or deletion of the transgene in each of the independent lines obtained. Animals were handled according to the French guidelines for animal care.
Figure 1.
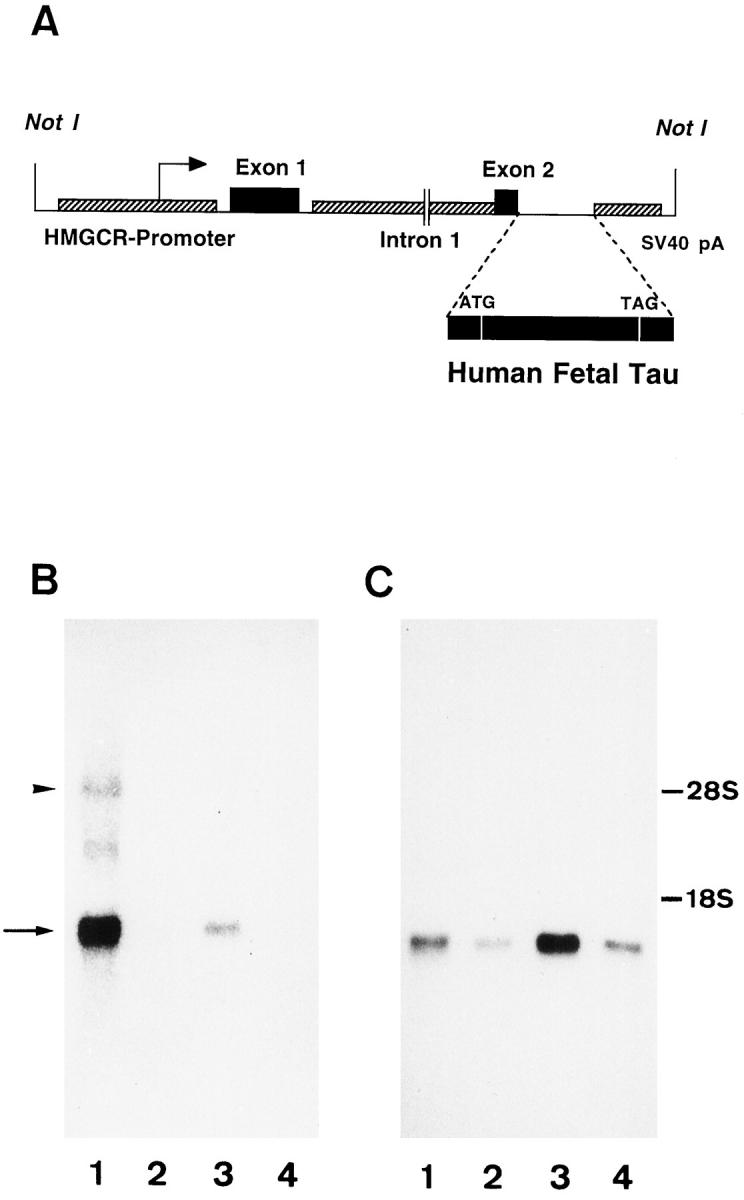
A: Structure of the hybrid gene used for the generation of transgenic mice expressing human fetal tau. B and C: Northern blots of total RNA from a transgenic mouse probed with the human tau cDNA probe (B) or the GAPDH probe (C). The transgenic tau mRNA (arrow in B) is strongly expressed in brain but is also detected in heart. The endogenous mouse tau mRNA (arrowhead in B) is poorly recognized by the human tau probe. Lane 1 , brain; lane 2, liver; lane 3, heart; lane 4, kidney.
RNA Analysis
Total RNA was isolated from adult murine kidney, liver, heart, and brain by the guanidium thiocyanate-phenol-chloroform method. 28 Aliquots of 20 μg of total RNA per lane were electrophoresed on a 1% (w/v) agarose gel under denaturing conditions. After migration, the RNA was transferred to a nylon membrane and cross-linked to the membrane with UV light and during a 2-hour incubation at 80°C. After prehybridization in 50% (w/w) formamide, 5× Denhardt’s solution, 5× SSC, 0.1% sodium dodecyl sulfate (SDS), and 100 μg of salmon DNA/ml at 42°C for 3 hours, the membrane was hybridized for 45 hours at 42°C with 32P-labeled probe generated by means of random priming of the fetal human tau cDNA. Washings were performed 2 times with 2× SSC and 0.1% SDS for 15 minutes at room temperature. Stripped membranes were rehybridized overnight with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe under the same experimental conditions.
Preparation of Brain Homogenates and Tau Proteins
The brains of controls and transgenic mice were homogenized on ice in a buffer containing 50 mmol/L Tris, 10 mmol/L EDTA, and 100 mmol/L sodium chloride, supplemented with protease inhibitors (1 mmol/L phenylmethylsulfonylfluoride, 25 μg/ml leupeptin, 25 μg/ml pepstatin A) and phosphatase inhibitors (10 mmol/L sodium pyrophosphate, 20 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate). Homogenates were solubilized additionally by adding sample buffer for SDS-polyacrylamide gel electrophoresis to give a final concentration of 2% (w/v) SDS.
Tau proteins were extracted from brain homogenates by treatment with 2.5% (v/v) perchloric acid for 60 minutes. Insoluble proteins were eliminated by centrifugation (20 minutes, 20,000 × g), and the resulting supernatant was dialyzed against 50 mmol/L Tris, 1 mmol/L dithiothreitol, 0.1 mmol/L phenylmethylsulfonylfluoride. Tau proteins were dephosphorylated by treatment with Escherichia coli alkaline phosphatase (1 hour at 67°C, 18 U/ml). 29
Western Blot Analysis
Tissue samples (100 μg of proteins/lane) were run on 10% (w/v) polyacrylamide SDS gels at pH 8.8 with stacking gels of 4% (w/v) polyacrylamide at pH 6.8 and then transferred on nitrocellulose using a semidry electroblotter (Ancos, Hoejby, Denmark). For immunoblotting, the nitrocellulose sheets were blocked in semifat dry milk (10% w/v in Tris-buffered saline) and incubated successively with the primary antibodies and with peroxidase-conjugated secondary antibodies (Amersham, Buckinghamshire, UK). Blots were revealed using the enhanced chemiluminescence detection system (Amersham).
Antibodies
A panel of antibodies was used to study the expression, the distribution, and the phosphorylation status of tau in transgenic animals (Table 1) ▶ . The B19 antibody is a rabbit polyclonal antibody raised against bovine tau protein, 30 reacting with all human and rodent tau isoforms. The TP20 antibody is a polyclonal antibody raised against a synthetic peptide corresponding to amino acids 32 to 41 of human tau; 30 this antibody was found to react with human tau but not with mouse tau. The BR10 polyclonal antibody was raised to a synthetic peptide corresponding to amino acids 84 to 101 of human tau (longest isoform); this sequence is present in the insert 2 found in the amino domain of some adult-type tau isoforms but not in the fetal tau isoform.
Table 1.
Immunoreactivity of the Human Transgenic Tau
| Antibody to tau | Epitope | Somatodendritic | Astrocytes |
|---|---|---|---|
| B19 | Tau (human, mouse)* | ++ | +++ |
| BR10 | 84–101 (human, mouse)* | − | − |
| TP20 | 32–41 (human)* | ++ | +++ |
| AT180 | Thr231 (P) | ++ | +++ |
| AT270 | Thr181 (P) | ++ | +++ |
| AD2 | Ser396/404 (P) | −/+ | +++ |
| PHF-1 | Ser396/404 (P) | −/+ | +++ |
| 12E8 | Ser262/356 (P) | −/+ | ++ |
| tau-1 | Ser199/202 (NP) | ++ | +++ |
| Alz50 | 7–9 and 312–342† | ++ | +++ |
| AT8 | Ser202/Thr205 (P) | − | − |
| AP422 | Ser422 (P) | − | − |
| AT10 | Thr212/Ser214 (P) | − | − |
| TG3 | ‡ | − | − |
The immunoreactivity was studied with antibodies requiring a phosphorylated epitope (P), an unphosphorylated epitope (NP), with phosphorylation-independent antibodies (*), with antibodies requiring a conformational epitope (†), or a conformational and phosphorylated epitope (‡). The numbers refer to the position of amino acids in the longest human tau isoform. The immunoreactivity was estimated on a semiquantitative scale: −, absent; −/+, very weak; +, moderate; ++, strong; +++, very strong.
The monoclonal antibodies AT8, AT180, AT270, AT10 (Innogenetics, Belgium) recognize tau phosphoepitopes; they are specific for tau phosphorylated at Ser202 and Thr205 (AT8), 31 Thr231 (AT180), Thr181 (AT270), and Thr212/Ser214 (AT10). 29,32 The tau monoclonal antibodies PHF-1 (kindly provided by Dr. P. Davies and S. Greenberg, New York) 33 and AD2 (kindly provided by Dr. A. Delacourte and Monsanto, France) 34 are specific for tau phosphorylated at Ser396 (and to less extent at Ser404). The tau monoclonal antibodies 12E8 (kindly provided by Dr. P. Seubert, Athena Neuroscience, San Francisco) and AP422 (kindly provided by Dr. M. Hasegawa and M. Goedert, Cambridge, UK) are specific for tau phosphorylated at Ser262/356 and Ser422, respectively. 35,36 The tau monoclonal antibody TG-3 (provided by Dr. P. Davies, New York) recognizes a conformation- and phosphorylation-dependent epitope requiring phosphorylation of Thr231. 37 The tau monoclonal antibody Alz50 (provided by Dr. P. Davies) recognizes a conformational epitope requiring both an N-terminal segment (including amino acids 7–9) and a C-terminal fragment. 38,39 The tau-1 monoclonal antibody (Boehringer Mannheim, Mannheim, Germany) recognizes an epitope that needs unphosphorylation of Ser199/202. 40
Additionally used antibodies were a monoclonal antibody to ubiquitin (clone MAB 1510, Chemicon, Temecula, CA), a monoclonal antibody to α-tubulin (clone DMIA, Sigma, St Louis, MO), a rabbit polyclonal antibody to Aβ(12–28) amyloid, 41 a rabbit polyclonal antibody to glial fibrillary acidic protein (GFAP), 42 and a rabbit polyclonal antibody to the 150-kd neurofilament polypeptide (no. NA 1216, Affiniti, Exeter, UK).
Histochemical and Immunocytochemical Analysis in Photonic Microscopy
To assess the brain distribution of transgenic tau during development, in adulthood and during aging, brains were dissected from transgenic mice at the following stages: 1, 7, and 15 days old, 6 weeks old, 6 to 7 months old, 10 to 12 months old, and 17 to 19 months old.
Tissue blocks were fixed by immersion with several types of fixatives: 10% (v/v) formalin, 4% (w/v) paraformaldehyde and 0.5% (v/v) glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4), and methacarn. Formalin- and methacarn-fixed samples were embedded in paraffin and cut on a sliding microtome at a thickness of 10 μm. Paraformaldehyde-fixed samples were cut in 50-μm-thick tissue sections on a vibratome.
The immunohistochemical labeling was performed using the avidin-biotin complex (ABC) method. Briefly, tissue sections were treated with H202 to inhibit endogenous peroxidase and incubated with the blocking solution (20% (v/v) normal goat serum in Tris-buffered saline (0.01 mol/L Tris, 0.15 mol/L NaCl, pH 7.4). After an overnight incubation with the diluted primary antibody, the sections were sequentially incubated with goat anti-rabbit or horse anti-mouse antibodies conjugated to biotin (Vector, Burlingame, CA) followed by the ABC complex (Vector). The peroxidase activity was revealed using diamininobenzidine as chromogen.
Double immunolabeling was performed using fluorescent markers. The first antibody was detected using an anti-rabbit or anti-mouse antibody conjugated to peroxidase followed by incubation with fluorescein tyramide (NEN, Boston, MA) and the second antibody detected using an anti-rabbit or anti-mouse antibody conjugated to biotin, followed by streptavidin conjugated to Alexa594 (Molecular Probes, Eugene, OR).
Formalin-fixed sections were also stained with the Gallyas silver-staining method; additional sections were stained with Congo red and examined under crossed polarization filters or stained with hematoxylin/eosin or with cresyl fast violet.
Immunolabeling in Electron Microscopy
Tissue blocks were quickly dissected and immediately fixed by immersion in 4% (w/v) paraformaldehyde and 0.5% (v/v) glutaraldehyde for 24 hours in 0.1 mol/L phosphate buffer at pH 7.4 for 90 minutes. For immunolabeling, 50-μm-thick tissue sections were cut on a vibratome, cryoprotected by incubation in 30% (w/v) sucrose, and frozen in isopentane cooled at −80°C. After thawing, the tissue sections were treated with 1% (w/v) sodium borohydride for 30 minutes and processed for immunolabeling with the ABC method (see above). After washing in Millonig’s buffer with 0.5% (w/v) sucrose for 24 hours, the tissue sections were postfixed in 2% (w/v) OsO4 for 30 minutes, dehydrated, and embedded in Epon. Semithin sections were stained with toluidine blue. Ultrathin sections were counterstained with uranyl acetate and lead citrate and observed with a Zeiss EM 809 at 80 kV.
Results
Generation of Transgenic Mice
To control the expression of the fetal human MAP tau, we used a construct with the promoter of the murine 3-hydroxy-methyl-glutaryl CoA reductase gene (HMG-CR). The HMG-CR promoter is a housekeeping-type promoter showing an ubiquitous expression pattern with a preferential expression in the brain. 43,44 The construct contained the 5′-flanking region, the first noncoding exon of the HMG-CR gene and the SV40 polyadenylation site (Figure 1A) ▶ . We obtained three independent transgenic lines with stable integration of the transgene. Southern blot analysis did not reveal any major rearrangement or deletion of the transgene (not shown). Only one out of the three lines transmitted the transgene to the offspring.
Northern Blotting
Northern blot analysis indicated a massive overexpression of human tau specific transcripts in the brain and to a lesser extent in heart (Figure 1B) ▶ . Endogenous tau mRNA was barely detectable; this faint signal is probably caused by the relatively poor homology between mouse adult and human fetal tau mRNA rather than to the low abundance of mouse mRNA as the latter was easily detected on a Northern blot probed with a mouse tau cDNA (not shown). The expression level of transgenic tau was about nine times higher in brain than in heart as we calculated from control hybridization with the GAPDH probe (Figure 1C) ▶ .
Western Blotting
Expression and Phosphorylation of the Human Transgenic Tau
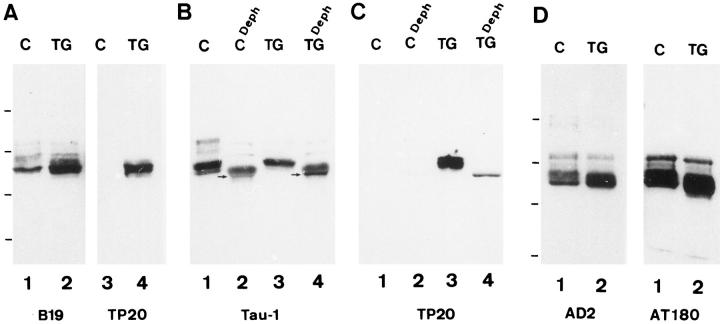
Brain homogenates of 6- to 12-month-old transgenic mice (line 23) and control littermate were analyzed by immunoblotting with several tau antibodies. In brain homogenates, several species with an apparent molecular mass between 48 and 68 kd were detected with the phosphorylation-independent B19 antibody, reacting with both mouse and human tau proteins (Figure 2A ▶ , lanes 1 and 2). The phosphorylation-independent and human specific TP20 antibody to tau exclusively detected a major band of 55 kd and two closely adjacent minor bands in brain homogenates of transgenic mice (Figure 2A ▶ , lanes 3 and 4).
Figure 2.
A: Western blots of brain homogenates from a control mouse (lanes 1 and 3) and from a transgenic mouse (lanes 2 and 4). The blots were incubated with the B19 anti-tau antibody (lanes 1 and 2) or the human-specific TP20 tau antibody (lanes 3 and 4). B and C: Western blots of brain homogenates from a control mouse (lane 1) and from a transgenic mouse (lane 3) and dephosphorylated tau proteins from the same control and transgenic mice (lanes 2 and 4). The blots were incubated with the tau-1 antibody (B) or the human-specific TP20 tau antibody (C). After dephosphorylation, four tau species are observed in control as well as in transgenic mice with the tau-1 antibody (lanes 2 and 4 in B), and a single tau species is detected with the human-specific TP20 antibody in the transgenic mouse (lane 4 in C). The TP20 antibody shows a slight cross-reactivity with alkaline phosphatase present in the control and transgenic dephosphorylated samples. The fastest migrating tau species (arrows in B) is more intensely labeled in transgenic mice. D: Western blots of brain homogenates from a control mouse (lane 1) and from a transgenic mouse (lane 2). The blots were incubated with the phosphorylation-dependent antibodies AD2 and AT180. C, control mouse; TG, transgenic mouse. Bars on the left indicate the position of molecular mass markers: 97.4 kd (phosphorylase b), 58.1 kd (catalase), 39.8 kd (alcohol dehydrogenase), 29.0 kd (carbonic anhydrase).
Tau proteins in brain homogenates are separated as a complex set of species reflecting both the expression of several isoforms and the presence of differentially phosphorylated species. To separate more precisely the different tau isoforms, tau proteins purified by perchloric acid treatment were dephosphorylated and subsequently analyzed by immunoblotting. After this treatment, the tau-1 and the B19 antibodies detected four main tau species in control and in transgenic mice, respectively, of molecular mass 46, 49, 56, and 64 kd (Figure 2B ▶ , lanes 2 and 4). Interestingly, in transgenic mice the fastest migrating tau species of 46 kd was much more intensely labeled. After dephosphorylation, a single species of 46 kd was detected by the TP20 antibody in transgenic mice (Figure 2C ▶ , lane 4) at the same level as the fastest migrating tau species detected by the tau-1 and the B19 antibodies. In consequence, we concluded that these intensely labeled 46-kd tau species were composed of a mixture of endogenous and transgenic fetal-type tau isoform. The close migration of mouse and human fetal-type tau isoforms is caused by the small difference in number of amino acids between mouse 45 and human tau. 26
Densitometric analysis of tau-1 immunoblots showed that the fastest migrating tau species of 46 kd account for 19% of total tau species in control mice and for 33% of total tau in transgenic mice; this increase is caused by the expression of transgenic tau proteins.
The phosphorylation-dependent tau antibodies AT180, AT270, and AD2 reacted with both endogenous and transgenic tau proteins (Figure 2D) ▶ . The tau-1 and 12E8 tau antibodies reacted with the endogenous tau but did not discriminate clearly the transgenic tau bands.
Immunocytochemical Localization of the Transgenic Human Tau Protein
We investigated the general distribution of the human transgenic tau with the TP20 antibody that reacts specifically with human tau. The transgenic tau was expressed in brain at all developmental stages studied, ie, from 1 day old through adulthood. In most of the examined cases, the expression of the transgenic tau was widespread and detected in cortical areas, hippocampus, caudatoputamen, thalamus, cerebellum, and the brainstem (Figure 3) ▶ . A labeling with the TP20 antibody was also observed in the spinal cord, Gasser ganglia, the sciatic nerve, and the nervous plexus of peripheral organs (eg, stomach and intestine) (not shown).
Figure 3.
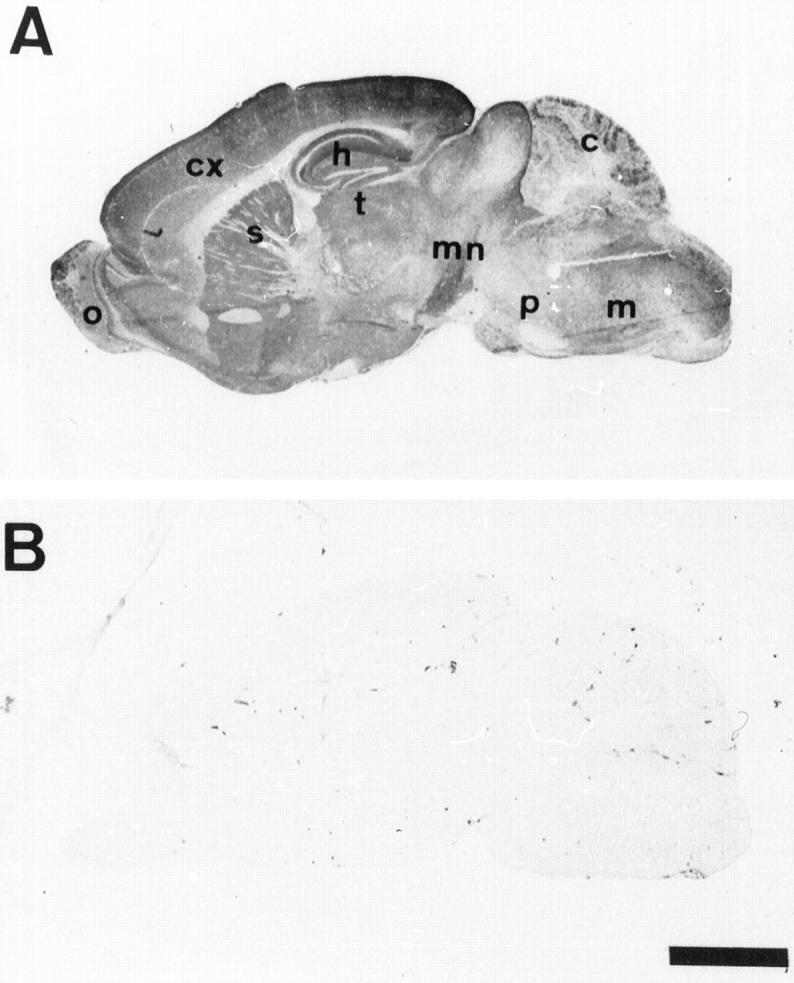
Immunolabeling with the human specific anti-tau antibody (TP20) on sagittal sections of the brain of a 6-month-old transgenic mouse (A) and a control mouse (B). The transgenic human tau protein has a widespread brain distribution. cx, cortex; h, hippocampus; c, cerebellum; o, olfactory bulb; s, striatum; t, thalamus; p, pons; m, medulla; mn, mesencephalon. Scale bar, 2 mm.
Neuronal Expression of the Transgenic Tau
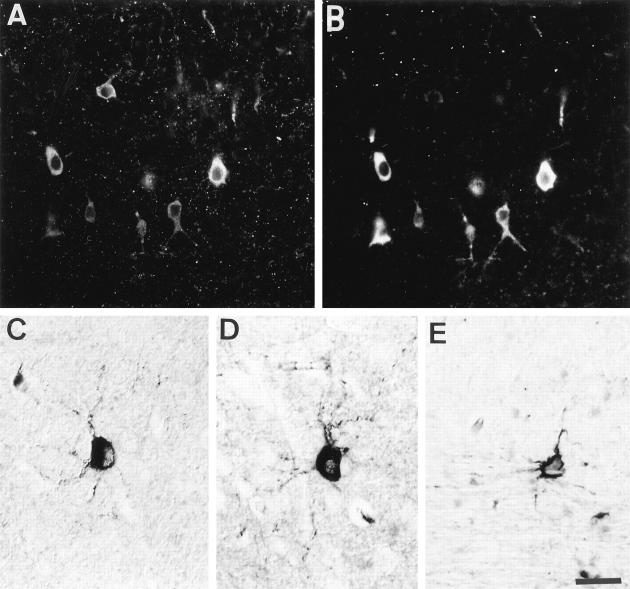
The human transgenic tau was detected by the TP20 antibody in cell bodies and dendrites of a population of neurons in many brain areas of adult transgenic mice. These neurons were more especially frequently observed in the hippocampus (Figure 4) ▶ , the cortex (most notably in the entorhinal cortex) (Figure 5) ▶ , and the striatum. They represented approximately 10% of the total neuronal population in the hippocampal pyramidal layer and the dentate gyrus. This intense somatodendritic labeling was confirmed using the B19 polyclonal anti-tau antibody reacting both with endogenous and transgenic tau. Such a significant somatodendritic labeling was not observed in control using the B19 antibody (Figure 6A) ▶ or the TP20 antibody (Figure 4D) ▶ .
Figure 4.
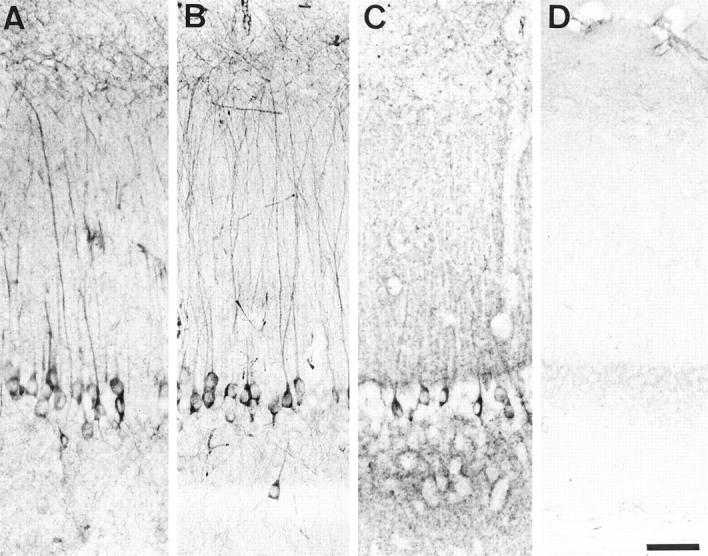
Immunolabeling of the hippoccampus (vibratome sections) in a 6-month-old transgenic mouse (A, B, and C) and in a control mouse (D). A and D: human specific TP20 antibody to tau; B: phosphorylation-dependent AT180 antibody to tau; C: tau-1 antibody to tau. A proportion of pyramidal cells in Ammon’s horn of the transgenic mouse show an accumulation of transgenic tau and AT180-immunoreactive phosphorylated tau in their somatodendritic compartment. Scale bar, 50 μm.
Figure 5.
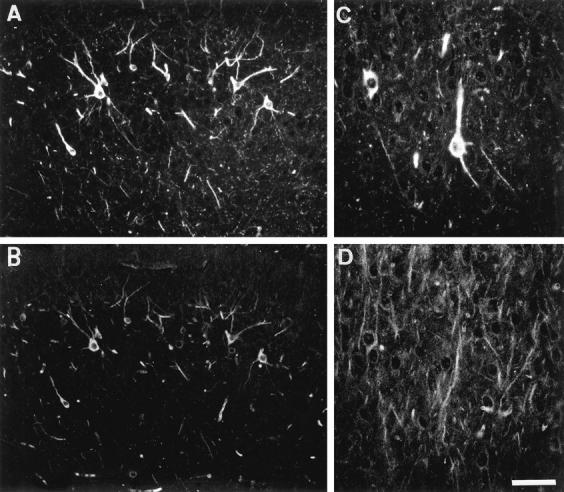
Immunolabeling on the entorhinal cortex in a 7-month-old transgenic mouse. A and B: Double immunolabeling with the human specific anti-tau antibody (TP20) (A) and with the phosphorylation-dependent AT180 anti-tau antibody (B). The same neurons show an accumulation of transgenic tau and of AT180-immunoreactive phosphorylated tau in their somatodendritic compartment. C and D: Double immunolabeling with the TP20 antibody (C) and with the α-tubulin antibody (D). The neurons expressing the transgenic tau do not show increased tubulin immunoreactivity. Scale bar, 40 μm (A and B), 20 μm (C and D).
Figure 6.
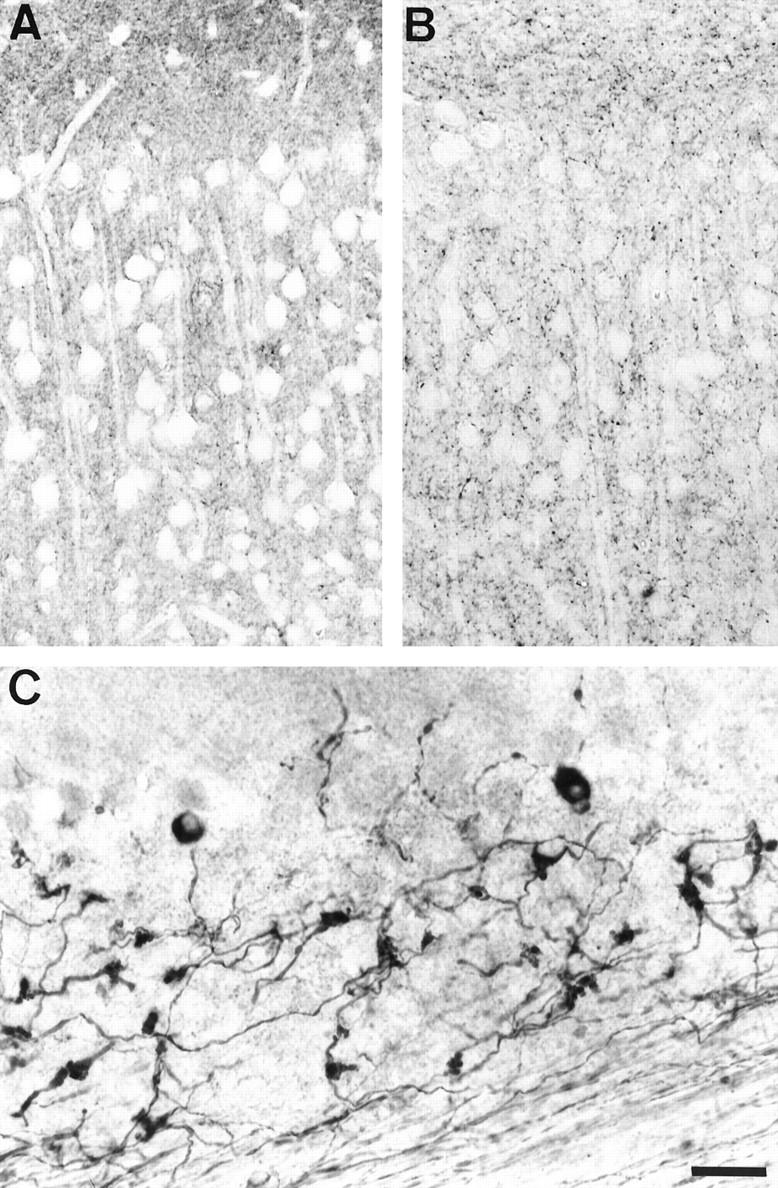
A and B: Immunolabeling of the cortex of an adult control mouse with the B19 anti-tau antibody (A) and of an adult transgenic mouse with the TP20 anti-tau antibody (B). The endogenous tau-immunoreactivity in the control mouse and the human tau-immunoreactivity in the transgenic mouse (in this selected area) are localized in axons terminals and not in cell bodies and dendrites. C: Immunolabeling of the cerebellum of an adult transgenic mouse with the TP20 antibody. The transgenic tau is abundant in axonal mossy fibers and in white matter axonal fibers. Two Purkinje cells also express the transgenic tau. Scale bar, 20 μm.
In the adult, the transgenic tau-immunoreactivity was also associated to axon terminals in gray matter (Figure 6B) ▶ and to axons in white matter tracts. Many small-caliber axons such as granule cell axons (running in the hippocampal stratum radiatum) and cerebellar mossy fibers (Figure 6C) ▶ were strongly immunoreactive. Double-immunolabeling with the SMI31 antibody (that detects phosphorylated heavy neurofilament polypeptide in axons) and the TP20 antibodies showed that only a proportion of axons was expressing the transgenic tau in many areas. For instance, approximately 5% of SMI31-positive axons in the sciatic nerve were TP20-positive (not shown). The neuropil and axonal labeling with the TP20 antibody appeared more generalized than the number of neurons with a somatodendritic labeling, suggesting that some axons expressing the transgenic tau originated from other neurons than those exhibiting a somatodendritic labeling.
Double immunolabeling with the TP20 antibody showed that neurons expressing the transgenic tau in their somatodendritic compartment were also labeled with the phosphorylation-dependent antibodies AT180 (Figure 5, A and B) ▶ , AT270 (see Figure 10, A and B ▶ ▶ ), tau-1, and the Alz50 antibody. They were not labeled with the other phosphorylation-dependent tau antibodies and with the BR10 tau antibody to insert 2 (absent from the transgenic tau) (Table 1) ▶ ; the BR10 antibody labeled, however, the neuropil and axons in mouse brain (not shown). No somatodendritic labeling with tau antibodies was observed in neurons that did not express the transgenic tau or in neurons in nontransgenic mice. The somatodendritic accumulation of the transgenic tau was not associated with modification of the tubulin immunoreactivity in the same cells as judged from double immunolabeling (Figure 5) ▶ , suggesting that the organization of the microtubule network (eg, bundling of microtubules) was not massively affected in these cells.
Figure 10.
Immunolabeling on the cortex of an adult transgenic mouse. A and B: Double immunolabeling with the TP20 antibody (A) and the phosphorylation-dependent AT270 antibody to tau (B). Neurons expressing the transgenic tau also show somatodendritic immunoreactivity with the AT270 antibody. C and D: Astrocyte-like cells showing a strong immunoreactivity with the antibodies B19 (C), AD2 (D), and 12E8 (E). Scale bar, 20 μm (A and B), 10 μm (C, D, and E).
The subcellular localization of the transgenic tau in neurons in the cerebellum and the hippocampus was studied by immunolabeling in electron microscopy with the TP20 antibody. Transgenic tau proteins were detected in a proportion of granule cells axons (parallel fibers) in the molecular layer of the cerebellar cortex (Figure 7B) ▶ . In these axons, the transgenic tau proteins were associated to microtubules. Transgenic tau proteins were also associated to dendritic microtubules in neurons showing a somatodendritic localization of transgenic tau, eg, in hippocampal pyramidal cells (Figure 7C) ▶ . In contrast, the transgenic tau present in cell bodies in the same cells was generally found in association with ribosome clusters or distributed more diffusely in the cytoplasm (Figure 7A) ▶ . An association of AT180-immunoreactive, phosphorylated tau species with dendritic microtubules was also observed (Figure 7D) ▶ .
Figure 7.
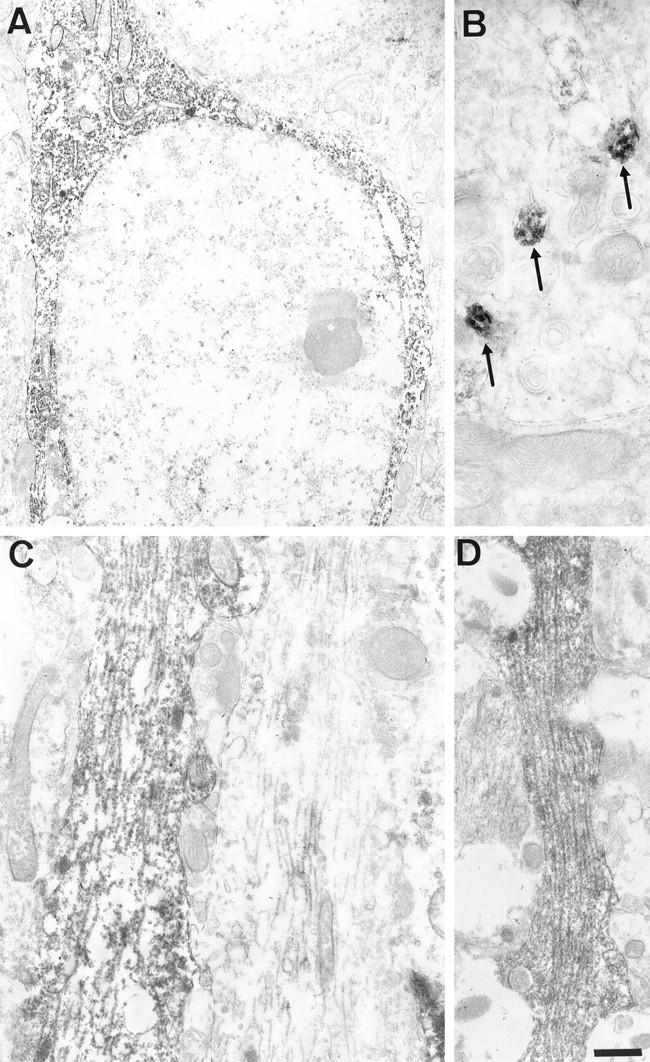
Immunolabeling in electron microscopy of the hippocampus (A, C, and D) and of the cerebellar molecular layer (B) in a transgenic mouse. A, B, and C: TP20 antibody. The hippocampal neuron in A shows a strong perikaryal labeling. The labeling is often associated with free ribosomes and with the rough endoplasmic reticulum. Some parallel fibers (granule cells axons) in B contain labeled microtubules (arrows) and are mixed with unlabeled parallel fibers. A labeled dendrite in C containing numerous labeled microtubules is adjacent to an unlabeled dendrite. D: AT180 antibody. A dendrite contains labeled microtubules. Scale bar, 1 μm (A); 750 nm (B); 500 nm (C and D).
Developmental Expression of Transgenic Tau
During development, the transgenic tau was already detected in neurons at the 1-day-old stage, in axons, in cell bodies, and in dendrites (Figure 8A) ▶ . At this developmental stage, a somatodendritic and axonal localization of endogenous tau was observed in all neurons in transgenic and nontransgenic animals. The phosphorylation-dependent tau antibodies (Figure 8, B and C) ▶ also detected this somatodendritic tau. During the next developmental stages, the somatodendritic tau immunoreactivity progressively disappeared in neurons that did not express the transgenic tau in transgenic and nontransgenic mice, whereas a population of neurons with a strong somatodendritic transgenic tau immunoreactivity persisted in transgenic animals up to adulthood (see above).
Figure 8.
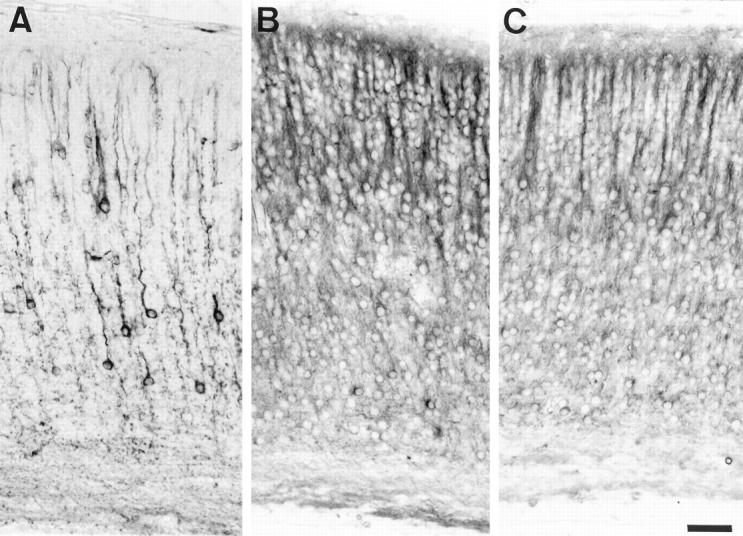
Immunolabeling of the cortex in 1-day-old transgenic mouse (A and B) and control mouse (C) with the human specific anti-tau antibody, TP20 (A), and the phosphorylation-dependent AT180 anti-tau antibody (B and C). The AT180-immunoreactive phosphorylated tau is present in the somatodendritic compartment of all neurons in control and transgenic animals, whereas the transgenic tau is expressed only in some neurons in transgenic animals. Scale bar, 20 μm.
The transgenic tau was not significantly expressed in neuroblasts localized in the ventricular zone in the developing brain or in the external granular layer in the developing cerebellum.
Expression of the Transgenic Tau in Non-Neuronal Cells
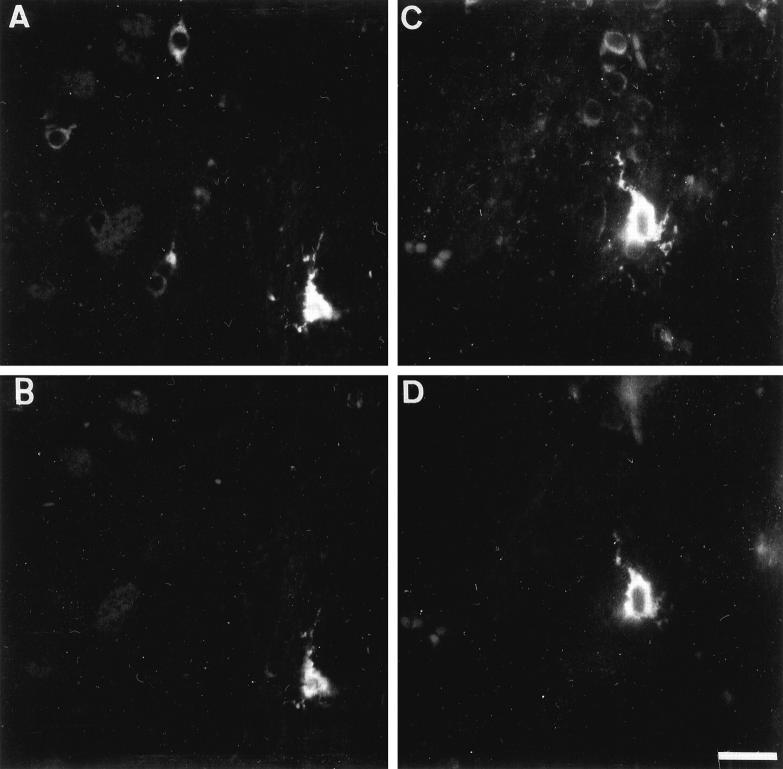
A few cells expressed very high levels of transgenic tau in their cell bodies and processes (Figures 9 and 10) ▶ ▶ . These cells were preferentially observed in white matter tracts but also in the cortical gray matter, caudate putamen, and the hippocampus. On a transversal brain tissue section of an adult transgenic mouse, approximately 5 to 10 of these cells were encountered. Double immunolabeling with TP20 and GFAP antibodies indicated that these cells were moderately GFAP positive (Figure 9, A and B) ▶ . Most of the intensely GFAP-positive astrocytes were, however, not expressing the transgenic tau protein. These GFAP-positive cells exhibited a strong immunoreactivity with phosphorylation-dependent tau antibodies AD2 and PHF-1 (ser 396/404), AT180 (thr 231), 12E8 (ser 262/356) (Figure 10E) ▶ , tau-1 (recognizing tau unphosphorylated on ser199/ser202) (Figure 10) ▶ , and Alz50, but not with antibodies AT8, AP422, AT10, and TG-3. The transgenic tau was also detected by immunocytochemistry in many peripheral organs, eg, in heart, thymus, lung, and stomach (not shown).
Figure 9.
A and B: Double immunolabeling of the striatum in a 7-month-old transgenic mouse with the human specific anti-tau antibody (TP20) (A) and the phosphorylation-dependent PHF-1 anti-tau antibody (B). Several striatal neurons express the transgenic tau but are not PHF-1 positive. A single cell localized in the corpus callosum express the transgenic tau and is strongly PHF-1 positive. C and D: Double immunolabeling with the anti-GFAP antibody (C) and the phosphorylation-dependent PHF-1 anti-tau antibody (D). The cells showing a strong PHF-1 immunoreactivity are GFAP positive. Scale bar, 20 μm (A and B), 12 μm (C and D).
Search for Neurofibrillary Lesions in Transgenic Mice
To investigate for the presence of neurofibrillary lesions in the transgenic animals, tissue sections of mice aged 6 to 7 months (n = 4), 10 to 12 months (n = 2), and 17 to 19 months (n = 3) were stained with Congo red and with the Gallyas silver staining method. Neither birefringent structures nor silver-stained intracellular inclusions were detected in these animals. The antiubiquitin antibody, that labels neurofibrillary tangles in Alzheimer’s disease, did not detect any fibrillary lesions in neurons, and the anti-Aβ antibody did not detect any amyloid deposit. We did not observe significant neuronal or glial cell degeneration in tissue sections stained with hematoxylin/eosin or with cresyl fast violet.
Discussion
The TG23 Transgenic Line Expresses a Phosphorylated Human Tau Transgenic Protein in Nervous Tissue
The tau transgenic line TG23 was generated using a cDNA encoding the shortest human tau protein placed under the control of the HMG-CR promoter. The human tau specific transcripts were highly expressed in the brain, and the transgenic tau protein was prominently expressed in neurons, although some non-neuronal cells also expressed the tau transgene. The level of transgenic tau (14% of total tau proteins) are superior to the level of transgenic tau (10%) obtained in the single tau transgenic line previously reported. 46 Our results demonstrate that the 3-hydroxy-methyl-glutaryl CoA promoter drives a very efficient transcription in the brain and in neurons, as shown previously in a transgenic line expressing a mutated form of the amyloid peptide precursor. 44
After dephosphorylation of tau proteins, we identified four tau species in mouse brain by immunoblotting. A similar pattern of four mouse tau species appearing after dephosphorylation has been reported in other studies. 46,47 However, an initial study 45 reported the isolation from mouse brain of only two tau cDNA encoding tau isoforms with three repeats, no amino-terminal inserts, but differing in their carboxyl-terminal domain. The detection of additional tau species by immunoblotting might reflect the persistence in some tau isoforms of constitutive phosphates or other posttranslational modifications affecting their electrophoretic mobilities. Alternatively, additional tau isoforms than those with three repeats and no amino-terminal inserts are expressed in adult mouse brain. In favor of the latter interpretation is our observation that the BR10 antibody raised to insert 2 present in the amino-terminal domain of some tau isoforms did react with mouse brain tissue.
In whole brain homogenates, transgenic tau proteins were detected as several bands, both by the human-specific antibody and by the phosphorylation-dependent tau antibodies AT180, AT270, and AD2. These different tau species most likely correspond to different phosphorylation states of the molecule because only a single species was observed after dephosphorylation. A similar observation was made in cultured cells transfected with a single tau cDNA. 48,49 The detection of the transgenic tau by phosphorylation-dependent antibodies demonstrates that it is an adequate substrate for protein kinases generating these phosphorylated epitopes, ie, the proline-directed kinases GSK-3β, MAPK, cdk5. 50-52 The detection of the transgenic tau with the phosphorylation-dependent tau antibody 12E8, although observed in some cells by immunocytochemistry (see below), was less clear on immunoblots of whole brain, suggesting that the pool of transgenic tau molecules phosphorylated on this site (Ser262/356) was relatively minor.
The Transgenic Tau Is Present in the Somatodendritic Compartment of a Subset of Mature Neurons
The abundance of the somatodendritic transgenic tau sharply contrasted with the very low level of tau immunoreactivity in the somatodendritic compartment in neurons nonexpressing the transgenic tau. In transgenic and nontransgenic animals, endogenous tau proteins were rather concentrated in axons and axons terminals as previously observed. 6,7 Several mechanisms have been implied in the axonal targeting of tau, ie, locally regulated microtubule binding, 9 selective stabilization of tau in axons, 10 and selective targeting of tau mRNAs at the basis of the axon. 8
The somatodendritic accumulation of transgenic tau might result from an overexpression of transgenic tau, leading to a saturation of mechanisms targeting tau to axons or an inability of these mechanisms to act on the transgenic tau. Furthermore, a cis-acting element located in the 3′-untranslated region of tau mRNA is implicated in tau mRNA targeting. 11 The absence of this signal in our transgene might lead to the observed high expression of transgenic tau in dendrites and cell bodies. However, we also detected the transgenic human tau protein in many axons. Therefore, we suggest that the axonal targeting of transgenic tau results from mechanisms independent of this cis-acting signal, for instance, operating at the protein level.
As previously reported, 53 we observed strong somatodendritic immunoreactivity for endogenous tau only during postnatal development. This endogenous somatodendritic immunoreactivity progressively decreased in the adulthood, whereas the transgenic tau immunoreactivity remained strong in the somatodendritic compartment. This suggests that brain maturation is associated to a maturation of mechanisms involved in tau compartmentalization and that these mechanisms are not operating efficiently on the transgenic tau.
By electron microscopy, the transgenic tau was found in association with axonal and dendritic microtubules. The transgenic tau is thus functionally able to interact in vivo with endogenous tubulin. Transfection of cells with tau constructs 54 often induces increased tubulin assembly in the form of microtubule bundles. In neurons expressing the transgenic tau, we did not observe increased tubulin immunoreactivity or obvious microtubule bundling. In addition, most of the transgenic tau accumulating in the cell body was not associated to microtubule bundles. This suggests that the relative concentrations of tubulin and transgenic tau might not be in the range needed for strong nucleation of microtubule bundles. The transgenic tau in cell bodies might also represent a pool of tau molecules functionally unable to bind to tubulin.
Altogether, these results indicate that the transgenic tau expressed in these mice is physiologically functional but that it shows aberrant intracellular targeting.
Similarities Between Pretangle Stage and Accumulation of Transgenic Tau in Cell Bodies
Several reports have shown that the three repeat tau isoform, although not unique, 55 is predominant into PHF. 56-58 It seemed thus appropriate to overexpress this type of tau isoform in a transgenic line, to favor its potential aggregation into PHF-like structures. However, the accumulation of human tau in cell bodies and dendrites of neurons and in astrocytes did not lead to the formation of neurofibrillary tangles or PHF, as we monitored with silver staining, Congo red staining, and immunolabeling in electron microscopy. PHF-like filaments can be generated in vitro using truncated tau molecules 59 or full-length tau molecules in presence of glycosaminoglycans 60 or RNA. 61 It is possible that high concentrations of tau sustained in these in vitro models but not in the present in vivo model could drive more easily its assembly into PHF-like filaments.
The accumulation of transgenic tau documented in the present transgenic line shows nevertheless similarities with the somatodendritic accumulation of phosphorylated tau observed at the pretangle stage preceding the formation of neurofibrillary tangles in Alzheimer’s disease. 24,25 The somatodendritic transgenic tau was detected by the Alz50 antibody, which labels the somatodendritic domain of neurons at early stages of neurofibrillary degeneration. 25 Alz50 recognizes a conformational epitope on tau, 38,39 and it has been suggested that monomeric tau accumulating at early stages of neurofibrillary degeneration undergoes a conformational alteration leading to its recognition by Alz50. Two phosphorylation-dependent antibodies (AT180, AT270) detected the somatodendritic transgenic tau, and four phosphorylation-dependent antibodies (AT180, AT270, PHF-1, 12E8) detected the transgenic tau expressed in astrocytes. The tau phosphoepitopes recognized by these antibodies have been all identified in PHF-tau proteins. 19,62 The antibodies PHF-1 and 12E8 are specific for tau phosphorylated at Ser396 and at Ser262, respectively. 33,35 The binding of tau to microtubules has been reported to be significantly reduced by phosphorylation at Ser396 4 and to be strongly reduced by phosphorylation at Ser262 (localized in the first repeat), 63 and it has been suggested that this would be responsible for the absence of binding of PHF-tau to microtubules. A proportion of tau molecules are phosphorylated on these two sites also in fetal and adult normal tau. 35,64,65 The phosphorylation of transgenic tau at Ser262 and Ser396 in astrocytes in the present transgenic line might contribute to a reduction in its ability to bind to microtubules and to stabilize them.
The mechanisms responsible for the higher phosphorylation of the transgenic tau in some astrocytes might result from differential expression or activities of protein phosphatases or protein kinases in neurons and astrocytes. Alternatively, a massive expression of the transgene in these glial cells could lead to a shift in the equilibrium between kinases and phosphatases activities. Although we did not detect neurofibrillary lesions in these astrocytes, it is noteworthy that astrocytes have a potential for generating these lesions as neurofibrillary lesions have been observed in astrocytes in progressive supranuclear palsy, 66 more rarely in Alzheimer’s disease 67 and in familial multiple system tauopathy. 68
In neurons affected by early changes preceding the formation of neurofibrillary tangles in human brain 69 or in aged sheep, 70 it was reported that abnormal tau proteins are associated with ribosomes. We similarly observed an association of the transgenic tau immunoreactivity to ribosomes in cell bodies, an additional similarity with early stages of neurofibrillary degeneration. These results are also compatible with the hypothesis that the pretangle stage in Alzheimer’s disease results from disturbances of mechanisms involved in the cellular compartmentalization of tau.
A single transgenic line of mice expressing the longest human tau isoform has been reported. 46 A somatodendritic localization of transgenic tau proteins, without formation of neurofibrillary tangles, was also observed in this previous line, but only a subset of these cells showed immunoreactivity with the phosphorylation-dependent tau antibodies AT8 and PHF-1. Glial cells expressing a high level of phosphorylated transgenic tau were not observed in this previous line, possibly because the neuron-specific thy-1 promoter drove the expression of the transgenic tau. The phosphorylated tau epitopes that we have detected are localized on amino acid sequences common to the different tau isoforms. Thus the additional phosphorylated epitopes generated in our transgenic mice, rather than being related to the type of tau isoform, might be related to the type of transgene construct and its level of expression.
Filamentous inclusions, but not neurofibrillary tangles, have also been observed in lamprey neurons microinjected with plasmids driving expression of tau. 71 A model of transgenic lines expressing the amyloid precursor protein has been reported to show an immunoreactivity for some tau phosphoepitopes in neurites closely associated with Aβ-amyloid deposits. 72 Neurons in the latter transgenic line do not however exhibit a somatodendritic accumulation of phosphorylated tau as we have observed in the present tau transgenic line. Other transgenic lines expressing mutant amyloid peptide precursor or presenilin and exhibiting cellular lesions 73-76 have not been reported to show changes in tau compartmentalization or phosphorylation.
Phosphorylation generating other phosphorylated epitopes and/or conformational epitopes not yet detected in tau transgenic lines might prove to be critical for the final formation of PHF-tau proteins and neurofibrillary lesions. Such sites are the epitope of antibody AT10, requiring simultaneous phosphorylation of Thr212 and Ser214, 32 the epitope of antibody AP422, depending on phosphorylation of Ser422 36 and the conformational epitope of antibody TG3. 37 Additional experimental studies aimed at the generation of the Alzheimer-type neurofibrillary lesions will include experimental modulation of the activities of protein kinases/phosphatases and cross-breeding experiments with animals expressing other key-molecules involved in the pathogenesis of Alzheimer’s disease.
Our results indicate nevertheless that manipulation of tau expression and intracellular targeting in a transgenic model is sufficient per se to affect tau compartmentalization, phosphorylation, and conformation at least in part as it is observed in pathological conditions.
Acknowledgments
The authors thank Dr. K. Kosik for providing the fetal human tau cDNA and Drs. P. Davies, A. Delacourte, M. Hasegawa, M. Goedert, and P. Seubert for providing the antibodies Alz50, PHF-1, AD2, AP422, and 12E8. We appreciate the excellent technical assistance of A.M. Couck and N. Touchet and the photographic work of J.L. Conreur.
Footnotes
Address reprint requests to Dr. Jean-Pierre Brion, Laboratory of Pathology and Electron Microscopy, Université Libre de Bruxelles, School of Medecine, 808, route de Lennik, Bldg C-10, 1070 - Brussels, Belgium.
Supported by grants from the Belgian Fonds de la Recherche Scientifique Médicale (3.4507.95) to J. P. Brion and from the Queen Elisabeth Medical Foundation to J. N. Octave. J. N. Octave is Senior Research Associate of the Belgian Fonds National de la Recherche Scientifique. This work was also partially supported by the BioAvenir program financed by the French Ministry of Research, the French Ministry of Industrie, and Rhône-Poulenc S.A.
References
- 1.Weingarten MD, Lockwood AH, Hwo SH, Kirschner MW: A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 1975, 72:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delacourte A, Buée L: Normal and pathological tau proteins as factors for microtubule assembly. Int Rev Cytol 1997, 171:167-224 [DOI] [PubMed] [Google Scholar]
- 3.Lindwall G, Cole RD: Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 1984, 259:5301-5306 [PubMed] [Google Scholar]
- 4.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 5.Lovestone S, Hartley CL, Pearce J, Anderton BH: Phosphorylation of tau by glycogen synthase kinase-3β in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 1996, 73:1145-1157 [DOI] [PubMed] [Google Scholar]
- 6.Binder LI, Frankfurter A, Rebhun I: The distribution of tau in the mammalian central nervous system. J Cell Biol 1985, 101:1371-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brion JP, Guilleminot J, Couchie D, Nunez J: Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience 1988, 25:139-146 [DOI] [PubMed] [Google Scholar]
- 8.Litman P, Barg J, Rindzoonski L, Ginzburg I: Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron 1993, 10:627-638 [DOI] [PubMed] [Google Scholar]
- 9.Kanai Y, Hirokawa N: Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron 1995, 14:421-432 [DOI] [PubMed] [Google Scholar]
- 10.Hirokawa N, Funakoshi T, Sato-Harada R, Kanai Y: Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J Cell Biol 1996, 132:667-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behar L, Marx R, Sadot E, Barg J, Ginzburg I: cis-acting signals and trans-acting proteins are involved in tau mRNA targeting into neurites of differentiating neuronal cells. Int J Dev Neurosci 1995, 13:113-127 [DOI] [PubMed] [Google Scholar]
- 12.Migheli A, Butler M, Brown K, Shelanski ML: Light and electron microscope localization of the microtubule-associated tau protein in rat brain. J Neurosci 1988, 8:1846-1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couchie D, Fages C, Bridoux AM, Rolland B, Tardy M, Nunez J: Microtubule-associated proteins and in vitro astrocyte differenciation. J Cell Biol 1985, 101:2095-2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feany MB, Dickson DW: Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol 1996, 40:139-148 [DOI] [PubMed] [Google Scholar]
- 15.Brion JP, Passareiro H, Nunez J, Flament-Durand J: Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol (Brux) 1985, 95:229-235 [Google Scholar]
- 16.Delacourte A, Defossez A: Alzheimer’s disease: tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci 1986, 76:173-186 [DOI] [PubMed] [Google Scholar]
- 17.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM: Microtubule-associated protein tau: a component of Alzheimer paired helical filaments. J Biol Chem 1986, 261:6084-6089 [PubMed] [Google Scholar]
- 18.Kosik KS, Joachim CL, Selkoe DJ: The microtubule-associated protein, tau, is a major antigenic component of paired helical filaments in Alzheimer’s disease. Proc Natl Acad Sci USA 1986, 83:4044-4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y: Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 1995, 270:823-829 [DOI] [PubMed] [Google Scholar]
- 20.Goedert M, Trojanowski JQ, Lee VMY: Tau protein, and the neurofibrillary pathology of Alzheimer’s disease. Wasco W Tanzi RE eds. Molecular Mechanisms of Dementia. 1997, :pp 199-218 Humana Press Inc, Totowa, New Jersey, [Google Scholar]
- 21.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B: Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998, 95:7737-7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD: Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998, 43:815-825 [DOI] [PubMed] [Google Scholar]
- 23.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, De Graaff E, Wauters E, Van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393:702-705 [DOI] [PubMed] [Google Scholar]
- 24.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM: Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 1989, 477:90-99 [DOI] [PubMed] [Google Scholar]
- 25.Braak E, Braak H, Mandelkow E-M: A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol (Berl) 1994, 87:554-567 [DOI] [PubMed] [Google Scholar]
- 26.Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VMY, Lee G: Epitopes that span the tau molecules are shared with paired helical filaments. Neuron 1988, 1:817-825 [DOI] [PubMed] [Google Scholar]
- 27.Hogan B, Constantini F, Lacey E: Manipulating the Mouse Embryo, a Laboratory Manual. 1987. Cold Spring Harbor Laboratory, Cold Spring Harbor
- 28.Chromczynski P, Sacchi N: Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 29.Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, Cras P: Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein. Biochem J 1994, 301:871-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brion JP, Hanger DP, Bruce MT, Couck AM, Flament-Durand J, Anderton BH: Tau in Alzheimer neurofibrillary tangles: N- and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem J 1991, 273:127-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goedert M, Jakes R, Vanmechelen E: Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995, 189:167-170 [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann R, Lee VMY, Leight S, Varga I, Otvos L, Jr: Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry 1997, 36:8114-8124 [DOI] [PubMed] [Google Scholar]
- 33.Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM-Y: Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 1994, 39:669-673 [DOI] [PubMed] [Google Scholar]
- 34.Buée-Scherrer V, Condamines O, Mourton-Gilles C, Jakes R, Goedert M, Pau B, Delacourte A: AD2, a phosphorylation-dependent monoclonal antibody directed against tau proteins found in Alzheimer’s disease. Mol Brain Res 1996, 39:79-88 [DOI] [PubMed] [Google Scholar]
- 35.Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GVW, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ, Lee VM-Y: Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem 1995, 270:18917-18922 [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa M, Jakes R, Crowther RA, Lee VMY, Ihara Y, Goedert M: Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett 1996, 384:25-30 [DOI] [PubMed] [Google Scholar]
- 37.Jicha GA, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P: A conformation-, and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem 1997, 69:2087-2095 [DOI] [PubMed] [Google Scholar]
- 38.Carmel G, Mager EM, Binder LI, Kuret J: The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem 1996, 271:32789-32795 [DOI] [PubMed] [Google Scholar]
- 39.Jicha GA, Bowser R, Kazam IG, Davies P: Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res 1997, 48:128-132 [DOI] [PubMed] [Google Scholar]
- 40.Biernat J, Mandelkow E-M, Schröter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, Mandelkow E: The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J 1992, 11:1593-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brion JP, Couck AM, Bruce M, Anderton BH, Flament-Durand J: Synaptophysin and chromogranin A immunoreactivities in senile plaques of Alzheimer’s disease. Brain Res 1991, 539:143-150 [DOI] [PubMed] [Google Scholar]
- 42.Lowenthal A, Flament-Durand J, Karcher D, Noppe M, Brion JP: Glial cells identified by anti-α albumin in human pineal gland. J Neurochem 1982, 38:863-865 [DOI] [PubMed] [Google Scholar]
- 43.Gautier C, Mehtali M, Lathe R: A ubiquitous mammalian expression vector, pHMG, based on a housekeeping gene promoter. Nucleic Acids Res 1989, 17:8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czech C, Delaère P, Macq AF, Reibaud M, Dreisler S, Touchet N, Schombert B, Mazadier M, Mercken L, Theisen M, Pradier L, Octave JN, Beyreuther K, Tremp G: Proteolytical processing of mutated human amyloid precursor protein in transgenic mice. Mol Brain Res 1997, 47:108-116 [DOI] [PubMed] [Google Scholar]
- 45.Lee G, Cowan N, Kirschner M: The primary structure and heterogeneity of tau protein from mouse brain. Science 1988, 239:285-288 [DOI] [PubMed] [Google Scholar]
- 46.Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, Bürki K, Goedert M: Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 1995, 14:1304-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larcher JC, Boucher D, Ginzburg I, Gros F, Denoulet P: Heterogeneity of Tau proteins during mouse brain development and differentiation of cultured neurons. Dev Biol 1992, 154:195-204 [DOI] [PubMed] [Google Scholar]
- 48.Gallo J-M, Hanger DP, Twist EC, Kosik KS, Anderton BH: Expression and phosphorylation of a three-repeat isoform of tau in transfected non-neuronal cells. Biochem J 1992, 286:399-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medina M, De Garcini EM, Avila J: The role of tau phosphorylation in transfected COS-1 cells. Mol Cell Biochem 1995, 148:79-88 [DOI] [PubMed] [Google Scholar]
- 50.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH: Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filaments epitopes and neuronal localization of the kinase. Neurosci Lett 1992, 147:58-62 [DOI] [PubMed] [Google Scholar]
- 51.Drewes G, Lichtenberg-Kraag B, Döring F, Mandelkow E-M, Biernat J, Goris J, Dorée M, Mandelkow E: Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J 1992, 11:2131-2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishiguro K, Omori A, Sato K, Tomizawa K, Imahori K, Uchida T: A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett 1991, 128:195-198 [DOI] [PubMed] [Google Scholar]
- 53.Brion JP, Octave JN, Couck AM: Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience 1994, 63:895-909 [DOI] [PubMed] [Google Scholar]
- 54.Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisawa M, Masaki T, Kirokawa N: Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol 1989, 109:1173-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakes R, Novak M, Davison M, Wischik CM: Identification of 3- and 4-repeat tau isoforms within the PHF in Alzheimer’s disease. EMBO J 1991, 10:2725-2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori H, Hamada Y, Kawaguchi M, Honda T, Kondo J, Ihara Y: A distinct form of tau is selectively incorporated into Alzheimer’s paired helical filaments. Biochem Biophys Res Commun 1989, 159:1221-1226 [DOI] [PubMed] [Google Scholar]
- 57.Greenberg SG, Davies P, Schein JD, Binder LI: Hydrofluoric acid-treated τ-phf proteins display the same biochemical properties as normal τ. J Biol Chem 1992, 267:564-569 [PubMed] [Google Scholar]
- 58.McLaughlin L, Zemlan FP, Dean GE: Identification of microtubule-associated protein tau isoforms in Alzheimer’s paired helical filaments. Brain Res Bull 1997, 43:501-508 [DOI] [PubMed] [Google Scholar]
- 59.Wille H, Drewes G, Biernat J, Mandelkow E-M, Mandelkow E: Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol 1992, 118:573-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA: Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 1996, 383:550-553 [DOI] [PubMed] [Google Scholar]
- 61.Kampers T, Friedhoff P, Biernat J, Mandelkow EM: RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett 1996, 399:344-349 [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, Ihara Y: Protein sequence and mass spectrometric analyses of tau in the Alzheimer’s disease brain. J Biol Chem 1992, 267:17047-17054 [PubMed] [Google Scholar]
- 63.Biernat J, Gustke N, Drewes G, Mandelkow E-M, Mandelkow E: Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 1993, 11:153-163 [DOI] [PubMed] [Google Scholar]
- 64.Matsuo ES, Shin R-W, Billingsley ML, Van DeVoorde A, O’Connor M, Trojanowski JQ, Lee VM-Y: Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron 1994, 13:989-1002 [DOI] [PubMed] [Google Scholar]
- 65.Liu WK, Yen SH: The state of phosphorylation of normal adult brain τ, fetal τ, and τ from Alzheimer paired helical filaments at amino acid residue Ser262. J Neurochem 1996, 66:1131-1139 [DOI] [PubMed] [Google Scholar]
- 66.Nishimura M, Namba Y, Ikeda K, Oda M: Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci Lett 1992, 143:35-38 [DOI] [PubMed] [Google Scholar]
- 67.Yamazaki M, Nakano I, Imazu O, Terashi A: Paired helical filaments and straight tubules in astrocytes: an electron microscopic study in dementia of the Alzheimer type. Acta Neuropathol (Berlin) 1995, 90:31-36 [DOI] [PubMed] [Google Scholar]
- 68.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B: Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA 1997, 94:4113-4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papasozomenos SC: Tau protein immunoreactivity in dementia of the Alzheimer type: II. Electron microscopy and pathogenetic implications. Lab Invest 1989, 60:375-389 [PubMed] [Google Scholar]
- 70.Nelson PT, Saper CB: Ultrastructure of neurofibrillary tangles in the cerebral cortex of sheep. Neurobiol Aging 1995, 16:315-323 [DOI] [PubMed] [Google Scholar]
- 71.Hall GF, Yao J, Lee G: Human tau becomes phosphorylated and forms filamentous deposits when overexpressed in lamprey central neurons in situ. Proc Natl Acad Sci USA 1997, 94:4733-4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B: Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA 1997, 94:13287-13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E: Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373:523-527 [DOI] [PubMed] [Google Scholar]
- 74.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang FS, Cole G: Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 1996, 274:99-102 [DOI] [PubMed] [Google Scholar]
- 75.Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS: Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 1997, 19:939-945 [DOI] [PubMed] [Google Scholar]
- 76.Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PLR, Siedlak SL, Tabaton M, Perry G: Amyloid-β deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 1998, 70:2212-2215 [DOI] [PubMed] [Google Scholar]