Abstract
This case-control study was designed to identify factors associated with long-term survival. We examined two groups of patients with epithelial ovarian cancer, one group of long-term survivors (>5 years) and one group of short-term survivors (<2 years), for levels of expression of p53 and p27KIP1 proteins (as both proteins have been shown to be independent prognostic markers in tumors other than ovary) and the relationship with patient survival. Our findings show that p27KIP1 expression, in contrast to p53 expression, is positively associated with long-term survival in univariate analysis (P = 0.001), in analyses stratified by residual disease (P = 0.02) or performance status (P = 0.02), the two strongest prognostic factors for ovarian cancer, as well as multivariate analysis (P = 0.002) adjusting simultaneously for age, tumor stage, residual disease, performance status, and grade of differentiation. Therefore, immunostaining for levels of p27KIP1 expression may have potential as a new prognostic factor in the management of ovarian cancer.
Epithelial ovarian cancer is the fifth leading cause of cancer deaths among women in the United States. 1 The long-term prognosis for women with advanced epithelial ovarian cancer remains poor, with only a minority of women surviving more than 5 years. The efficacy of cancer chemotherapy for ovarian cancer is limited by the development of resistance to chemotherapy. 2 Advanced epithelial ovarian cancer (stages IIC, III, and IV) shows greater than 70% initial response rates to combination chemotherapy with platinum-based compounds, but the tumors often recur, becoming resistant to chemotherapy.
Platinum-based chemotherapy induces apoptosis in tumor cells, and reduced susceptibility to apoptosis has been proposed as a major mechanism responsible for resistance to chemotherapy. 3-5 One study found that ovarian cell lines transfected with a p53-expressing plasmid showed increased resistance to cisplatinum, suggesting that p53 expression may decrease the sensitivity of ovarian cell lines to chemotherapy. 4 The p53 tumor suppressor gene is one of the most frequently mutated genes in human cancer, including approximately 50% of ovarian carcinomas. 6,7 As mutant p53 protein is relatively stable compared with wild-type p53 protein and accumulates within the cell nucleus, immunohistochemistry is frequently used to screen for the presence of p53 gene mutations. Studies have shown contradictory results as to the prognostic significance of p53 protein accumulation as a marker of poor prognosis in ovarian cancer. 6-9
Several recent studies have shown that the cell-cycle-dependent kinase inhibitor p27KIP1 is a promising molecular marker of poor prognosis in several cancers. 10-18 The p27KIP1 gene is a member of the cip/kip family of cyclin-dependent kinase inhibitors (CKIs), which includes p21, p27KIP1, and p57. 19 CKIs bind to cyclin/cyclin-dependent kinase (CDK) complexes, consequently blocking progression through the cell cycle. The cip/kip family member p27KIP1 regulates progression from G1 into S phase by binding and inhibiting the cyclin E/CDK2 complex necessary for entry into S phase. Levels of p27KIP1 are elevated in the quiescent cell but decrease in the cell re-entering the cell cycle, allowing progression into the S phase. Levels of p27KIP1 protein are regulated at the post-transcriptional level through degradation by the ubiquitin pathway. 20 A decrease or absence of p27KIP1 protein expression in breast, colorectal, gastric, small-cell lung, and prostate carcinomas has shown a strong association with poor prognosis. 10-17 In ovarian cancer, however, p27KIP1 protein expression has not been examined for clinical significance in predicting patient survival.
The objective of our study was to identify factors associated with long-term survival. We investigated whether levels of expression of p53 or p27KIP1 proteins in ovarian tumors (as both proteins have been shown to be independent prognostic markers in tumors other than ovary) were associated with patient survival. We examined two groups of patients with ovarian cancer: one group of long-term survivors (>5 years) and one group of short-term survivors (<2 years). Our findings show that p27KIP1 expression, in contrast to p53 expression, is a new prognostic marker, in addition to tumor stage, grade, residual disease, and performance status, for assessing survival outcomes among women with ovarian cancer.
Materials and Methods
Study Design
Because our objective was to identify factors associated with long-term survival among women with ovarian cancer, we designed a study to compare long-term survivors (>5 years) with short-term survivors (<2 years), using a case-control design. The cutoff points were chosen before the start of the study. We decided a priori to exclude stage I tumors because of the high long-term survival rates associated with these tumors. To increase comparability of the two groups with respect to known prognostic factors, we restricted eligibility to age <80 years at date of surgery, tumor stage II or III (tumor stage I with predictable long-term survival status was excluded) according to the International Federation of Gynecologists and Obstetricians, a pathological diagnosis of Mullerian epithelial carcinoma, and a Karnofsky performance status of 2 or less. Patients who had undergone surgery at the Tisch Hospital Center of New York University Medical Center (NYUMC) in the years 1981 to 1990 were retrospectively evaluated for eligibility using the Gynecologic Tumor Registry maintained at NYUMC. A total of 66 eligible patients (33 long-term and 33 short-term survivors) were identified. Archival paraffin blocks were retrieved for 54 of 66 (82%) of the cases: 30 long-term survivors and 24 short-term survivors. Classification of ovarian tumors among long-term survivors consisted of 20 papillary serous, 6 adenocarcinomas, 3 mucinous, and 1 clear cell, whereas those tumors among short-term survivors were classified as 15 papillary serous, 4 adenocarcinomas, 3 mucinous, 2 clear cell, and 1 endometrioid. None of the patients had received chemotherapy or radiation therapy before surgical resection. All of the patients were treated with a platinum-based chemotherapeutic regimen according to current treatment protocols at the time. Blocks selected by the pathologist showed greater than 75% malignant epithelium with minimal necrosis by evaluation of hematoxylin and eosin (H&E)-stained slides. The patient survival data were unknown to the pathologist.
Immunohistochemistry
Six-micron sections were cut from formalin-fixed paraffin-embedded tissue blocks and mounted on Superfrost/Plus glass slides. The sections were deparaffinized in xylene and rehydrated. For antigen retrieval and detection of p53 and p27KIP1, the sections were heated in a microwave oven for a total of 30 minutes (three cycles of 10 minutes each) in 10 mmol/L sodium citrate buffer at pH 6.0. Endogenous peroxidase activity was eliminated by preincubation in 2% H2O2 in methanol for 30 minutes followed by three washes in phosphate-buffered saline (PBS). The sections were stained using standard streptavidin-biotin complex immunoperoxidase methods (Histostain-SP kit, Zymed Laboratories, South San Francisco, CA) on a Ventana ES machine (Ventana Medical Systems, Tucson, AZ). The primary antibodies used were as follows: for p53 (BP53–12, BioGenex, San Ramon, CA), a mouse monoclonal antibody used at 1:100, and for p27KIP1 (K25020, Transduction Laboratories, Lexington, KY), a mouse monoclonal antibody used at 1:400. All antibodies were diluted in PBS containing 1% normal rabbit serum. Peroxidase activity was localized with chromogen 3,3′-diaminobenzidine tetrachloride in 0.5 mmol/L Tris buffer. The slides were counterstained with Delafield’s hematoxylin. Normal ovarian tissue served as a negative control for p53 and as a positive control for p27KIP1 immunostaining.
Assessment of Immunostains
Samples were coded, the immunostaining was assessed at a multihead microscope, and the percentage of immunostained cells was determined by a minimum of three viewers. For both p53 and p27KIP1 immunostaining, only a distinct brown nuclear staining was scored as positive. A cutoff value of <5% immunopositive tumors cells was considered as negative, and greater than 5% was considered as positive. For p27KIP1, positive samples were scored according to the frequency of immunopositive cells as 5% to 25%, 26% to 50%, 51% to 75%, and >75% cells immunopositive. Samples from patients with <50% p27KIP1-positive tumor cells were considered low expressors, whereas those with >50% p27KIP1-positive tumor cells were considered high expressors according to the published convention. 11,13
Statistical Analysis
The associations between categorical prognostic factors (including p27KIP1 and p53 expression) and survival status were assessed using the χ2 test or Fisher’s exact test. Ordered categorical variables were assessed using χ2 test for trend, and continuous variables were assessed using the Mann-Whitney test. Similar methods were used to assess the associations of p27KIP1 or p53 expression with known prognostic factors for ovarian cancer. To evaluate whether p27KIP1 expression was an independent prognostic factor of survival status, we conducted analyses stratified by residual disease and performance status, the two strongest prognostic factors for ovarian cancer. Finally, multivariate analysis using a logistic regression model was used to assess the prognostic value of p27KIP1 expression, adjusting simultaneously for age, stage, residual disease, performance status, and grade of differentiation.
Results
Clinical Data
The univariate analysis of clinical parameters of the two groups of ovarian cancer patients is summarized in Table 1 ▶ . As expected, short-term survivors tended to be older and to have more advanced stage at diagnosis, more residual disease at surgery, a performance status of 1 or more, and tumors that were more poorly differentiated than long-term survivors. Of the five prognostic factors evaluated in univariate analysis, only extent of residual disease and performance status showed significant differences between the two groups. Of 20 patients in the short-term survivor group, 12 (65%) had >2 cm of residual disease after initial tumor debulking surgery, compared with only 5 of 28 (18%) of the patients in the long-term survivor group (P = 0.001). Performance status at the time of diagnosis of ovarian cancer also was associated with survival. In the short-term survivor group, 23 of 24 (96%) had a performance status of 1 or more, whereas 17 of 30 (57%) of the long-term survivors had a performance status of 1 (P = 0.001).
Table 1.
Univariate Analysis of Patient Characteristics of Long-Term and Short-Term Survivors
| Variable | Long-term survivors (n = 30) | Short-term survivors (n = 24) | P value |
|---|---|---|---|
| Median age (years) | 52.6 (37.5–78.0) | 60.8 (31.8–77.7) | NS |
| Race | |||
| Caucasian | 27 (90%) | 21 (88%) | NS |
| Other | 3 (10%) | 3 (13%) | |
| Tumor stage | |||
| IIC | 6 (20%) | 1 (4%) | NS |
| III | 24 (80%) | 23 (96%) | |
| Residual disease | |||
| Microscopic | 13 (46%) | 3 (13%) | 0.001 |
| <2 cm | 10 (36%) | 5 (22%) | |
| >2 cm | 5 (18%) | 15 (65%) | |
| Performance status | |||
| 0 | 13 (43%) | 1 (4%) | 0.001 |
| 1 | 17 (57%) | 20 (83%) | |
| 2 | 0 | 3 (13%) | |
| Differentiation | |||
| Well/moderate | 12 (43%) | 7 (19%) | NS |
| Poor | 18 (60%) | 17 (71%) |
NS, not significant.
Immunohistochemistry for p53 and Patient Survival
Levels of p53 protein were assessed by immunohistochemistry, and tumors were scored as negative or positive for protein accumulation. The frequency for p53 protein accumulation was similar in both groups of women with ovarian cancer (Table 2) ▶ . Positive immunostaining for p53 protein was detected in 14 of 23 (61%) tumors from short-term survivors and in 18 of 30 (60%) tumors from long-term survivors. There was no association between p53 protein expression and survival status in this study (P value was not significant (NS)).
Table 2.
Univariate Analysis of p53 and p27 Expression as Prognostic Markers
| Variable | Long-term survivors | Short-term survivors | P value |
|---|---|---|---|
| p53 expression | |||
| Negative | 12 (40%) | 9 (39%) | NS |
| Positive | 18 (60%) | 14 (61%) | |
| p27 expression | |||
| Negative | 0 | 4 (17%) | 0.001 |
| <25% | 3 (10%) | 3 (13%) | |
| 26–50% | 4 (14%) | 13 (54%) | |
| 51–75% | 4 (14%) | 4 (17%) | |
| >75% | 18 (62%) | 0 | |
| High expressors | 22 (76%) | 4 (17%) | 0.001 |
| Low expressors | 7 (24%) | 20 (83%) |
NS, not significant.
Immunohistochemistry for p27KIP1 Expression
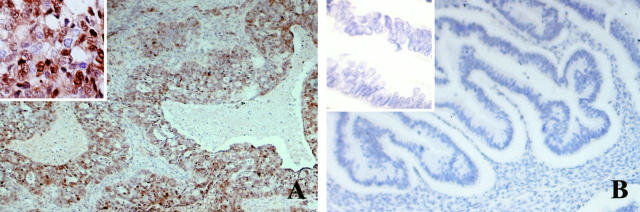
Figure 1 ▶ shows p27KIP1 immunostaining in representative serous carcinomas from a long-term (Figure 1A) ▶ and a short-term survivor (Figure 1B) ▶ . In serous carcinomas, the most common type of ovarian tumor, a tumor from a long-term survivor (Figure 1A) ▶ representative of a high expressor shows >50% p27KIP1-positive nuclei in epithelial cells, whereas the tumor from a short-term survivor is completely negative for p27KIP1 staining in the malignant epithelium (Figure 1B) ▶ .
Figure 1.
Representative histological sections showing p27KIP1 immunostaining. A: Serous carcinoma from a long-term survivor representative of a high expressor shows >50% nuclear reactivity for p27KIP1 in malignant epithelial cells. B: Serous carcinoma from a short-term survivor shows absence of nuclear reactivity for p27KIP1 in malignant epithelial cells. Magnification, ×100 and ×400 (inset).
Immunohistochemistry for p27KIP1 and Patient Survival
Samples from patients with <50% p27KIP1-positive tumor cells were considered low expressors, whereas those with >50% p27KIP1-positive tumor cells were considered high expressors according to the published convention. 11,13 A strong association was found between p27KIP1 protein expression and survival status (Table 2) ▶ . Among the short-term survivors, 20 of 24 (83%) of the patients were low expressors for p27KIP1, whereas among long-term survivors, only 7 of 29 (24%) of the patients were low expressors for p27KIP1 (P = 0.001).
Expression Levels of p27KIP1: Association with Other Prognostic Factors
Decreased levels of p27KIP1 protein were associated with other poor prognostic factors for ovarian cancer. As shown in Table 3 ▶ , when comparing low versus high expressors for p27KIP1, no significant difference was observed with regard to age, race, stage of disease, grade of tumor differentiation (poor versus moderate), or status of p53 protein expression. However, decreased levels of p27KIP1 protein expression were associated more frequently with patients having >2 cm of residual disease after initial tumor debulking surgery. In this study 15 of 26 (58%) low expressors for p27KIP1 had significant residual disease compared with 5 of 24 (21%) high expressors (P = 0.02). Levels of p27KIP1 expression also were correlated with performance status. Among patients with low expression levels of p27KIP1, 23 of 27 (85%) had a performance status of 1 or more, in contrast to 16 of 26 (62%) of the patients with high expression levels of p27KIP1 (P = 0.02).
Table 3.
Univariate Analysis of p27 Expression and Prognostic Factors
| Variable | p27 | P value | |
|---|---|---|---|
| High expressors | Low expressors | ||
| Age (years) | |||
| ≤55 | 14 (54%) | 12 (44%) | NS |
| >55 | 12 (46%) | 15 (56%) | |
| Race | |||
| Caucasian | 23 (89%) | 24 (89%) | NS |
| Other | 3 (12%) | 3 (11%) | |
| Tumor stage | |||
| IIC | 4 (15%) | 3 (11%) | NS |
| III | 22 (85%) | 24 (89%) | |
| Residual disease | |||
| Microscopic | 10 (42%) | 6 (38%) | 0.02 |
| <2 cm | 9 (38%) | 5 (19%) | |
| >2 cm | 5 (21%) | 15 (58%) | |
| Performance status | |||
| 0 | 10 (39%) | 4 (15%) | 0.02 |
| 1 | 16 (62%) | 20 (74%) | |
| 2 | 0 | 3 (11%) | |
| Differentiation | |||
| Well/moderate | 11 (42%) | 8 (30%) | NS |
| Poor | 15 (58%) | 19 (70%) | |
| p53 expression | |||
| Negative | 11 (44%) | 9 (33%) | NS |
| Positive | 14 (56%) | 18 (67%) |
NS, not significant.
Expression Level of p27KIP1 Is a New Prognostic Factor
To examine whether p27KIP1 protein expression was independently associated with patient survival we conducted analyses stratified by residual disease and performance status (Table 4) ▶ . The association between p27KIP1 levels of expression and survival status remained significant after stratification and was present in each of the strata. Among those patients with >2 cm of residual disease, 13 of 15 (87%) of the short-term survivors were low expressors for p27KIP1, in contrast to 2 of 5 (40%) of the long-term survivors (P = 0.001). Similarly, among patients with performance status of 1, 16 of 20 (80%) women were low expressors of p27KIP1 among short-term survivors compared with 4 of 16 (25%) among long-term survivors (P = 0.001). The association of p27KIP1 expression with survival status also remained significant (P = 0.002) after adjusting simultaneously for age, tumor stage, residual disease, performance status, and grade of differentiation in multivariate analysis. However, due to the small number of patients, confounding cannot be ruled out. Taken together, these results suggest that p27KIP1 expression may be an independent prognostic factor of survival in epithelial ovarian cancer.
Table 4.
Distribution of p27 Expression by Survival Status Stratified by Residual Disease and Performance Status
| Variable | Long-term survivors (n = 29) | Short-term survivors (n = 24) | P value for stratified test |
|---|---|---|---|
| Residual disease | |||
| Microscopic | |||
| p27 < 50% | 3 (23%) | 3 (100%) | |
| p27 > 50% | 10 (77%) | 0 | |
| <2 cm | |||
| p27 < 50% | 2 (22%) | 3 (60%) | 0.001 |
| p27 > 50% | 7 (78%) | 2 (40%) | |
| >2 cm | |||
| p27 < 50% | 2 (40%) | 13 (87%) | |
| p27 > 50% | 3 (60%) | 2 (13%) | |
| Performance status | |||
| 0 | |||
| p27 < 50% | 3 (23%) | 1 | |
| p27 > 50% | 10 (77%) | 0 | |
| 1 | |||
| p27 < 50% | 4 (25%) | 16 (80%) | 0.001 |
| p27 > 50% | 12 (75%) | 4 (20%) | |
| 2 | |||
| p27 < 50% | 0 | 3 (100%) | |
| p27 > 50% | 0 | 0 |
Discussion
The objective of our study was to identify factors associated with long-term survival. We asked whether some variable other than standard clinical prognostic factors would be predictive of survival. We selected two well characterized molecular markers, p53 and p27KIP1, for study as they have been associated previously with poor prognosis in many other cancers. 8,9-18 In this study, p27KIP1 expression was positively associated with long-term survival in univariate analysis and in analyses stratified by residual disease or performance status, the two strongest prognostic factors for ovarian cancer, as well as in multivariate analysis adjusting simultaneously for age, tumor stage, residual disease, performance status, and grade of differentiation These results suggest that p27KIP1 expression may have independent prognostic value. However, this study had a limited sample size and was restricted to patients whose survival was either long (>5 years) or short (<2 years) and did not include patients whose survival was intermediate (2 to 5 years). Cohort studies including larger numbers of epithelial ovarian cancer patients, and assessing the association of p27KIP1 with exact survival time after controlling for other prognostic factors, will be required to further assess the value of p27KIP1 as an independent prognostic factor of survival. Finally, our study was limited to stage II and III tumors, and additional studies will be needed to assess whether our results also apply to stage I and IV tumors.
All patients evaluated in this study received a platinum drug in combination with alkylating agents. There was no evidence from our own analysis (data not shown) that changes in these regimens over time significantly affected survival. 21-23 It will be interesting to examine in future studies whether the prognostic significance of p27KIP1 expression is also observed in patients receiving regimens containing taxanes, topoisomerase I inhibitors, and other new agents.
In this study, there was a positive correlation between high levels of p27KIP1 expression, survival >5 years, less residual disease, and better performance status. However, no correlation was observed between levels of p27KIP1 expression and tumor stage or the extent of differentiation of the tumor. A trend for low p27KIP1 expression and poorly differentiated colon, breast, gastric, and parathyroid tumors has been reported. 24-27 Additional studies will be required on larger numbers of ovarian tumors to determine whether levels of p27KIP1 expression are related to cellular differentiation.
Mutations of the p53 gene occur in approximately 50% of ovarian cancers. 6,28 Accumulation of p53 protein detected by immunohistochemical techniques has shown a close correlation with the presence of mutation in the p53 gene. 6,7,28,29 However, the relationship between p53 overexpression and survival has not been definitely established. Some studies have shown a trend toward poor survival in patients with p53 protein accumulation. 8,9,30-32 In contrast, there are several large studies that found no statistical association between the presence or absence of p53 gene mutations and patient survival. 6,7,28,33,34 The results of this study clearly demonstrate that p53 mutation and protein accumulation occurred with the same frequency in ovarian tumors from short- or long-term survivors and clearly supports the conclusion of Eltabbakh et al 34 that p53 overexpression is not an independent prognostic factor.
Recognized prognostic factors for ovarian cancer known to influence 5-year survival are tumor stage, amount of residual disease, performance status, and histological grade. 35 In addition, the molecular markers DNA ploidy and proliferative activity measured as Ki67 index have been associated with biologically more aggressive tumor growth. 36-38 In this report we show that levels of the cell-cycle-dependent kinase inhibitor p27KIP1 is a new prognostic factor associated with survival in ovarian cancer. The association of low to no expression levels of p27KIP1 with poor prognosis has been reported for breast, colorectal, gastric, Barrett’s esophageal, non-small-cell lung, and prostate carcinomas. 10-18 In several of these studies, no correlation between levels of p27KIP1 expression and cell proliferation measured as Ki67 index was observed. Other factors, such as the cell cycle control protein cyclin D1, have been postulated to regulate expression levels of p27KIP1 in a negative regulatory feedback loop. 24 In this regard, it is interesting to note that elevated levels of cyclin D1 have been detected in ovarian tumors and correlated with malignancy. 39 A similar association of cyclin D1 overexpression and malignancy has been reported for a number of different tumors, including breast, colorectal, and uterine. 40 Additional studies will be required on the ovarian tumors used in the current study to elucidate the role of cyclin D1 or other cell cycle regulators and their association with prognosis.
In summary, despite recent advances in treatment of ovarian cancer, no novel prognostic factor has been identified that might provide information related to long-term prognosis. Low levels of expression of the p27KIP1 protein show a consistent and strong association with poor prognosis in many types of tumors, including those derived from the ovarian surface Mullerian epithelium. As women in this study had Mullerian epithelial tumors with similar clinical features, use of a simple immunostaining technique for expression of p27KIP1 may have considerable potential as an independent prognostic indicator for the routine assessment and management of women with ovarian cancer.
Acknowledgments
We acknowledge our colleagues at New York University Medical Center for assisting in the study and management of the patients in this report. We thank S. Goswami, B. Rosenberg, and E. Ludwig for excellent technical assistance, and we are grateful to M. Pagano for helpful suggestions and for reading the manuscript.
Footnotes
Address reprint requests to Dr. James L. Speyer, Department of Medicine and Kaplan Comprehensive Cancer Center, New York University Medical Center, New York, NY 10016. E-mail: newcoe01@mcrcr.med.nyu.edu.
Supported by a core grant from NIH grant CA-16087 and The Gynecologic Research Fund of New York University Medical Center.
References
- 1.Lundis SH, Murray T, Bolden S, Wingo PA: Cancer statistics CA. Cancer J Clin 1998, 48:6-13 [DOI] [PubMed] [Google Scholar]
- 2.Thigpen JT, Vance RB, Khansur T: Second-line chemotherapy for recurrent carcinoma of the ovary. Cancer 1993, 71:1559-1564 [DOI] [PubMed] [Google Scholar]
- 3.Dive C, Hickman JA: Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer 1991, 65:192-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, Young LS: The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene 1995, 11:1217-1228 [PubMed] [Google Scholar]
- 5.Perego P, Giarola M, Righetti SC, Supino R, Caserini C, Delia D, Pierotti MA, Miyashita T, Reed JC, Zunino F: Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res 1996, 56:556-562 [PubMed] [Google Scholar]
- 6.Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, Clarke-Pearson DL, Iglehart JD, Bast RC, Berchuck A: Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res 1991, 51:2979-2984 [PubMed] [Google Scholar]
- 7.Kohler MF, Marks JR, Wiseman RW, Jacobs IJ, Davidoff AM, Clarke-Pearson DL, Soper JT, Bast RC, Berchuck A: Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst 1993, 85:1513-1519 [DOI] [PubMed] [Google Scholar]
- 8.Levesque MA, Katsaros D, Yu H, Zola P, Sismondi P, Giardina G, Diamandis EP: Mutant p53 protein overexpression is associated with poor outcome in patients with well or moderately differentiated ovarian carcinoma. Cancer 1995, 75:1327-1338 [DOI] [PubMed] [Google Scholar]
- 9.Klemi PJ, Pylkkanen L, Kiiholma P, Kurvinen K, Joensuu H: p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer 1995, 76:1201-1208 [DOI] [PubMed] [Google Scholar]
- 10.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta G, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 11.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nature Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 12.Tan P, Cady B, Wanner M, Worland P, Cukor B, Fiorentino M, Magi-Galluzzi C, Lavin P, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a, b) invasive breast carcinomas. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 13.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 14.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 15.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T: p27 expression and gastric carcinoma. Nature Med 1997, 3:593. [DOI] [PubMed] [Google Scholar]
- 16.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM: Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res 1998, 58:542-548 [PubMed] [Google Scholar]
- 17.Yang RM, Naitoh J, Murphy MJ, Wang H-J, Philipson J, DeKernion JB, Loda M, Reiter RE: Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol 1998, 159:941-945 [PubMed] [Google Scholar]
- 18.Singh SP, Lipman J, Goldman H, Ellis FH, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M: Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res 1998, 58:1730-1735 [PubMed] [Google Scholar]
- 19.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 20.Pagano M, Tam SW, Theodoras AM, Beer-Romano P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of ubiquitin-proteosome pathway in regulating the abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 21.Piccart MJ, Speyer JL, Wernz JC, Noumoff J, Beller U, Beckman M, Dubin N, Demopoulos R, Muggia F: Advanced ovarian cancer: three-year results of a (6–8 month), 2 drug cisplatin-containing regimen. Eur J Cancer Clin Oncol 1987, 23:631-641 [DOI] [PubMed] [Google Scholar]
- 22.Beller U, Speyer JL: Current issues in the evaluation and treatment of epithelial carcinoma of the ovary. Bottino JC Opfell RW Muggia FM eds. Experimental and Clinical Progress in Cancer Chemotherapy. 1985, :pp 235-263 Martinus Nijhoff, Boston [Google Scholar]
- 23.Speyer JL, Mandeli J, Hochster H, Runowicz C, Wadler S, Wallach R, Cohen C, Oette D, Sorich J, Demakos E, Gelpke L, Goldberg G, Bruckner HJ, Holland J: A Phase I trial of cyclophosphamide and carboplatinum combined with interleukin-3 in women with advanced-stage ovarian cancer. Gynecol Oncol 1995, 56:387-394 [DOI] [PubMed] [Google Scholar]
- 24.Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB: Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res 1998, 58:114-122 [PubMed] [Google Scholar]
- 25.Fredersdorf S, Burns J, Milne AM, Packham GP, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O’Hare MJ, Lu X: High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27kip1 and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 1997, 94:6380-6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasui W, Kudo Y, Semba S, Yokozaki H, Tahara E: Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn J Cancer Res 1997, 88:625-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd RV, Jin L, Qian X, Kulig E: Aberrant p27kip1 expression in endocrine and other tumors. Am J Pathol 1997, 150:401-407 [PMC free article] [PubMed] [Google Scholar]
- 28.Skomedal H, Kristensen GB, Abeler VM, Borresen-Dale A-L, Trope C, Holm R: TP53 protein accumulation and gene mutation in relation to overexpression of MDM2 protein in ovarian borderline tumours and stage I carcinomas. J Pathol 1997, 181:158-165 [DOI] [PubMed] [Google Scholar]
- 29.Skilling JS, Sood A, Niemann T, Lager DJ, Buller RE: An abundance of p53 null mutations in ovarian carcinoma. Oncogene 1996, 13:117-123 [PubMed] [Google Scholar]
- 30.Bosari S, Viale G, Radaelli U, Bossi P, Bonoldi E, Coggi G: p53 accumulation in ovarian carcinomas and its prognostic implications. Hum Pathol 1993, 24:1175-1179 [DOI] [PubMed] [Google Scholar]
- 31.Henriksen R, Strang P, Wilander E, Backstrom T, Tribukait B, Oberg K: p53 expression in epithelial ovarian neoplasms: relationship to clinical and pathological parameters, Ki-67 expression and flow cytometry. Gynecol Oncol 1994, 53:301-306 [DOI] [PubMed] [Google Scholar]
- 32.Rohlke P, Milde-Langosch K, Weyland C, Pichlmeier U, Jonat W, Loning T: p53 is a persistent and predictive marker in advanced ovarian carcinomas: multivariate analysis including comparison with Ki67 immunoreactivity. J Cancer Res Clin Oncol 1997, 123:496-501 [DOI] [PubMed] [Google Scholar]
- 33.Hartmann LC, Podratz KC, Keeney GL, Kamel NA, Edmonson JH, Grill JP, Su JQ, Karzmann JA, Roche PC: Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol 1994, 12:64-69 [DOI] [PubMed] [Google Scholar]
- 34.Eltabbakh GH, Belinson JL, Kennedy AW, Biscotti CV, Casey G, Tubbs RR, Blumenson LE: p53 overexpression is not an independent prognostic factor for patients with primary ovarian epithelial cancer. Cancer 1997, 80:892-898 [PubMed] [Google Scholar]
- 35.Nguyen HN, Averette HE, Hoskins W, Sevin BU, Penalver N, Steren A: National survey of ovarian carcinoma. VI. Critical assessment of current international federations of gynecology and obstetrics staging system. Cancer 1993, 72:3007-3011 [DOI] [PubMed] [Google Scholar]
- 36.Brescia RJ, Barakat RA, Beller U, Frederickson G, Suhrland MJ, Dubin N, Demopoulos RI: The prognostic significance of nuclear DNA content in malignant epithelial tumours of the ovary. Cancer 1990, 65:141-147 [DOI] [PubMed] [Google Scholar]
- 37.Diebold J, Suchy B, Baretton GB, Blasenbreu S, Meier W, Schmidt M, Rabes H, Lohrs U: DNA ploidy and MYC DNA amplification in ovarian carcinomas: correlation with p53 and bcl-2 expression, proliferative activity and prognosis. Virchows Arch 1996, 429:221-227 [DOI] [PubMed] [Google Scholar]
- 38.Garzetti GG, Ciavattini A, Goteri G, De Nictolis M, Stramazzotti D, Lucarini G, Biagini G: Ki67 antigen immunostaining (MIB 1 monoclonal antibody) in serous ovarian tumors: index of proliferative activity with prognostic significance. Gynecol Oncol 1995, 56:169-174 [DOI] [PubMed] [Google Scholar]
- 39.Barbieri F, Cagnoli M, Ragni N, Pedulla F, Foglia G, Alama A: Expression of cyclin D1 correlates with malignancy in human ovarian tumours. Br J Cancer 1997, 75:1263-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartkova L, Lukas J, Strauss M, Bartek J: Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene 1995, 10:775-777 [PubMed] [Google Scholar]