Abstract
CD95(Fas/APO-1)-ligand (CD95L) mediates apoptosis by trimerization of the CD95 receptor on the surface of sensitive cells. In vitro studies have shown CD95L expression mainly by activated T cells and suggested a role for CD95L in the regulation of immune responses. Little is known, however, about the cellular distribution of CD95L in situ in the normal human immune system. We investigated CD95L expression in tissue sections of the thymus, lymph node, spleen, tonsil, and gastrointestinal tract using in situ hybridization and two monoclonal antibodies. In all these organs, cells expressing CD95L message and protein were scarce and comprised scattered lymphocytes, rare nonlymphoid cells, and a subset of epithelioid endothelial cells. Surprisingly, a subset of plasma cells turned out to be the most prominent producers of CD95L, matching the reports on CD95L in myeloma cells. CD95L+ plasma cells were most numerous in the mucosa-associated lymphoid tissue. This also applied to acquired mucosa-associated lymphoid tissue in chronic gastritis in which CD95L+ plasma cells were found scattered in the lamina propria. Our data suggest that plasma cells as yet may be neglected modulators of immune responses.
The CD95(Fas/APO-1) ligand (CD95L) is a 40-kd type II membrane protein and member of the tumor necrosis factor superfamily of cytokines. 1,2 CD95L induces apoptosis in CD95-sensitive cells. The membrane-bound ligand may be cleaved from the cellular surface by metalloproteases giving rise to a soluble and functionally active form (sCD95L) of approximately 26 kd. 3,4
Although initially identified in activated T cells and T-cell lines, 4-7 CD95L is not a T-cell-restricted molecule. Reports on detection of CD95L in Sertoli cells of the testis 8 and various ocular cell types 9 resulted in the hypothesis of “immune-privileged tissues” in which inflammation may be prevented by CD95-induced apoptosis of infiltrating lymphocytes. 10,11 Expression of CD95L in immune-privileged organs like testis, brain, uterus, ovary, and prostate was subsequently reported also in mice. 12
As CD95L is being detected in an ever-growing number of malignant neoplastic cells and cell lines, 13-15 the concept of immunoprivileged sites has been extended to malignomas in which tumor cells may fight a CD95-mediated “counterattack” against tumor-invading lymphocytes and thus escape immune surveillance. 16
In addition to its possible role in maintaining such immunoprivileged sites, CD95L has been implicated in the regulation and down-modulation of immune processes. 17-19 Lpr/lpr and gld/gld mice that have defects in CD95 and CD95L function, respectively, develop lymphadenopathy because of accumulation of CD4−CD8− T cells. 20,21 Moreover, they bear autoreactive B cells and have elevated levels of immunoglobulins including anti-ssDNA and anti-dsDNA antibodies. 22 Thus, the CD95/CD95L system is also involved in the elimination of activated and potentially harmful B cells. 23
Here we present for the first time the identification and distribution of CD95L expressing cells in normal human lymphoid organs.
Materials and Methods
Cells and Tissues
The human T-leukemia cells, Jurkat, were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mmol/L HEPES (pH 7.2), and 2 mmol/L glutamine at 37°C with 5% CO2 in a humidified atmosphere. The cell viability was greater than 95% in each cell preparation used. To enhance CD95L expression, Jurkat cells were stimulated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA) and 0.5 μg/ml ionomycin (both from Sigma, St. Louis, MO). Cleavage of CD95L from the cell surface was inhibited by a 3-hour incubation of cells with metalloprotease inhibitor 1,10-phenanthroline (1.5 mmol/L) (Sigma).
Sf9 insect cells were purchased from Life Technologies, Inc. (Gaithersburg, MD) and cultured at 27°C in Grace’s insect medium (Life Technologies, Inc.) supplemented with 10% heat-inactivated fetal calf serum.
Nontransformed lymphocytic cells were obtained in a single case from a cervical cystic lymphangioma (hygroma) in a 2-month-old boy and then resected for diagnostic/therapeutic reasons. The cyst fluid was centrifuged and cytospin preparations of the pellet were done immediately following surgery.
Tissue from human tonsil (n = 7), lymph node (n = 6), spleen (n = 6), thymus (n = 7), stomach in chronic gastritis (n = 3), and appendix (n = 6) were surgically removed and snap frozen in liquid nitrogen and also fixed overnight in 4% neutral buffered formalin within 30 minutes of surgery. Frozen sections of approximately 5 μm were prepared and fixed in acetone for 10 minutes. Paraffin-embedded tissue samples were cut into sections of about 3 to 4 μm and processed as described.
Enrichment of Human Tonsillar Plasma Cells
Human tonsils (n = 2) were obtained from routine tonsillectomy, and cell suspensions were prepared by mincing the tissue and vigorous vortexing. After lysation of erythrocytes using hypotonic ammonium chloride buffer (0.15 mol/L NH4Cl, 0.01 mol/L KHCO3, 0.1 mmol/L EDTA), T lymphocytes were depleted by rosetting with 2-aminoethylisothiouronium pretreated sheep red blood cells and Ficoll-Hypaque (Pharmacia, Piscataway, NJ) gradient centrifugation. The enriched B cells and plasma cells were layered over Percoll (Biochrom, Berlin, Germany) step gradients in 15-ml conical tubes with 3 ml of 70% (1.085 g/ml), 60% (1.077 g/ml), 50% (1.067 g/ml), 40% (1.056 g/ml), and 30% (1.043 g/ml) Percoll. After centrifugation at 1200 × g for 15 minutes at 4°C, cells from the 30/40% Percoll interface turned out to contain most plasma cells. Cytocentrifugates were performed and air dried before immunofluorescence double staining.
Anti-CD95L mAb
The mouse anti-human CD95L mAb G247–4 (IgG1) was purchased from Pharmingen (San Diego, CA).
The mouse anti-hCD95L mAb 139 was developed in P. Krammer’s laboratory as previously described for rabbit anti-CD95L Ab. 3 Briefly, the 18-mer peptide HPSPPPEKKELRKVAHLC was conjugated to keyhole lympet hemocyanin by m-maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce, Rockford, IL) and mixed with complete Freund’s adjuvant. BALB/c mice were immunized with 100 μg of the conjugated peptide. The animals were boosted at biweekly intervals with half the dose of the same immunogen in incomplete Freund’s adjuvant and bled 7 to 8 days after each boost. Splenocytes were fused with the myeloma cells Ag8 and growing hybridomas were selected in hypoxanthine aminopterin thymidine medium. Supernatant of the hybridoma 139 reacted in enzyme-linked immunoabsorbent assay with the immunizing but not with an unrelated control peptide. This hybridoma was selected for additional studies and subcloned six times by limiting dilution. Isotyping performed using a mouse hybridoma subtyping kit (Boehringer Mannheim, Mannheim, Germany) showed that mAb 139 is an IgG1,κ. Mouse IgG were purified by affinity chromatography on protein G Sepharose (Pharmacia, Uppsala, Sweden) according to the manufacturer’s instructions. Purity was assessed by Coomassie staining of preparations separated by sodium dodecyl sulfate-polyacrylamide gel ectrophoresis. No differences in the reactivity were found between the hybridoma supernatant and the purified preparations. The hybridoma is stably producing mAb after more than 6 months of continuous in vitro culture.
Expression of Recombinant CD95L and TRAIL in Sf9 Insect Cells
The recombinant soluble human CD95L, mouse CD95L, and mouse TRAIL were expressed in Sf9 cells as previously described. 24,25 Briefly, recombinant baculoviruses were expanded to generate a stock virus by infecting Sf9 cells at a multiplicity of infection of 0.1. Infection of Sf9 cells for production of recombinant proteins was performed with a multiplicity of infection of 10. Cells were collected 4 to 5 days after infection, centrifuged at 1000 × g for 10 minutes, washed with phosphate-buffered saline (PBS), and stored as frozen pellets at −80°C. Deglycosylation was performed by treating Sf9 cells with tunicamycin (10 μg/ml) during infection (48 hours).
SDS-PAGE and Immunoblotting
SDS-PAGE and immunoblotting were performed as previously described. 3 Briefly, following SDS-PAGE, proteins were transferred to nitrocellulose membranes by semidry electroblotting. Membranes were blocked with 5% nonfat dried milk in PBS and then incubated overnight with the primary antibody. Membranes were washed five times with PBS/0.5% Tween 20 and incubated with horseradish-peroxidase-conjugated affinity-purified goat anti-mouse IgG or mouse anti-rabbit IgG Ab (Dianova, Hamburg, Germany) for 2 hours. Membranes were washed five times and the reaction developed using the ECL detection system (Amersham, Frankfurt, Germany).
Flow Cytometry
Flow cytometry was performed as previously described. 3 Briefly, 10 6 cells were incubated for 45 minutes on ice with purified mouse anti-CD95L mAb 139. Purified mAb MOPC21 (IgG1,κ) was used as isotype-matched negative control. Phyco-erythrin-labeled affinity purified F(ab)′2 goat anti-mouse IgG.Fc Ab (Dianova, Hamburg, Germany) was used as detecting reagent. Ten thousand events were acquired for each measurement. Only cell preparations with a viability higher than 95% were analyzed further. Results are expressed as histograms of fluorescence intensity or as percent positive cells.
In Situ Hybridization
CD95L mRNA was hybridized on dewaxed and rehydrated paraffin sections with the exon 4-specific digoxigenin-labeled human CD95L probe 1.APO-1L at 0.05 μmol/L as previously described. 26,27 After incubation with a gold-labeled sheep anti-digoxigenin antibody (Aurion, Wageningen, Netherlands), bound gold particles were visualized for light microscopy using a silver enhancement kit (Aurion). Sections were then slightly counterstained with 2% pyronine Y solution (Fluka, Buchs, Switzerland) and mounted with gelatin. Negative controls were carried out using 1) the unlabeled probe and 2) the digoxigenin-labeled CD95L promoter-specific oligonucleotide, Prom2, and did not yield any signal above background levels. 26
Immunohistochemistry and Immunofluorescence
Immunohistochemistry was carried out on cytospin preparations of cultured cells, frozen and paraffin sections. While acetone-fixed cytospins and frozen sections were directly incubated with mAb, formalin-fixed tissue had to be pretreated as follows. Before incubation with the anti-CD95L mAb G247–4, dewaxed and rehydrated paraffin sections were treated by microwaves (750 W) for 20 minutes in citrate buffer (pH 6.0) while they were digested for 5 minutes by pronase E (Sigma; 1 mg/ml in PBS) in the case of mAb 139.
Staining procedure was performed as described. 28 Briefly, cytospins/sections were incubated with mAb G247–4 (1:50) or mAb 139 (supernatant, 1:1; purified Ab, 1:2 to 1:5) in PBS for 60 minutes at room temperature. Binding sites of the primary antibody were visualized using either a biotin/streptavidin peroxidase system or the Dako EnVision™ kit (Dako, Copenhagen, Denmark) according to the manufacturer’s instructions. Cytospins/sections were then slightly counterstained with Harris’ hematoxylin and mounted with gelatin. Controls were done by omitting the primary antibody and, in part, by applying isotype-matched control mouse mAbs HEA125 and PR4.12 (Calbiochem, San Diego, CA).
For immunofluorescence, air-dried cytospin preparations were transferred into Tris buffer and incubated with protein blocking agent (Immunon, Pittsburgh, PA) for 10 minutes. This was followed by a 1-hour incubation with the mAb G247–4 (1:50) at room temperature. The cytospins were washed three times in Tris buffer and covered for 1 hour with fluorescein isothiocyanate-labeled rabbit antisera to human IgG, IgA, and IgM (1:50)(Immunon), respectively, together with a Cy3-conjugated goat anti-mouse IgG (1:50)(Jackson, West Grove, PA). After extensive washing in Tris, nuclei were stained by 4,6-diamidino-2-phenylindole for 3 minutes, and slides were mounted with PermaFluor aqueous mounting medium (Immunon). Controls were done by omitting the mAb G247–4 and the anti-human Ig antibody, respectively.
Results
Specificity of the New Anti-Human CD95L mAb 139
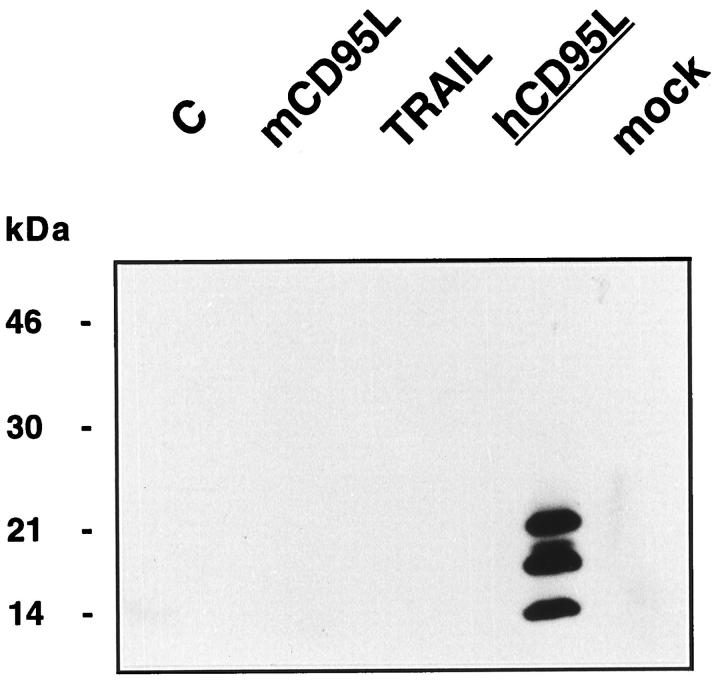
The mouse mAb 139, raised with a peptide of the extracellular region of human CD95L, reacted in immunoblotting with all of the differentially glycosylated forms of soluble human CD95L (Figure 1 ▶ , lane 4). Only the lower band (unglycosylated form) was present in immunoblot analysis of lysates of Sf9 cells expressing CD95L in the presence of tunicamycin (not shown). Conversely, mAb 139 did not react with whole cell lysates of Sf9 cells, of mock-infected Sf9 cells, or Sf9 cells expressing recombinant mouse CD95L or TRAIL. The latter were, however, detectable with specific Abs (not shown).
Figure 1.
Whole cell lysates of control Sf9 cells (C), Sf9 cells expressing recombinant mouse CD95L (mCD95L), TRAIL, soluble human CD95L (hCD95L), or mock-infected Sf9 cells (mock) were tested with mAb 139. Molecular weight markers are indicated. Horseradish-peroxidase-labeled goat anti-mouse IgG Fc Ab was used as the detecting reagent.
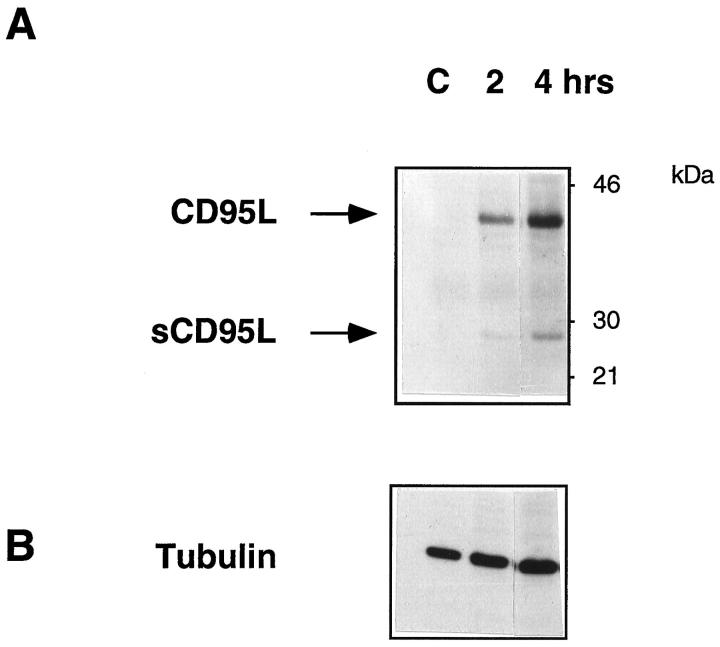
To assess the extent of reactivity of mAb 139 with human CD95L expressed by eukaryotic cells, Jurkat cells were analyzed. Figure 2A ▶ shows the reactivity of mAb 139 with whole lysates of Jurkat cells stimulated with plastic-coated anti-CD3 mAb, in immunoblotting. As expected, mAb 139 detected a protein of approximately 40 to 42 kd in 2 hour-activated Jurkat cells that increased in intensity at a later time point. It is of note that mAb 139 also detected the cell-associated soluble form of human CD95L at 26 to 27 kd as previously described with a polyclonal anti-CD95L Ab raised to a similar epitope. 3 Comparable loading of cell lysates in all lanes was verified by immunoblot analysis with an anti-tubulin mAb (Figure 2B) ▶ . No reactivity was found with purified mouse IgG (not shown).
Figure 2.
Whole cell lysates of Jurkat cells (C) cultured for 2 (lane 2) or 4 hours (lane 3) on plastic-coated anti-CD3 mAb were tested with mAb 139 (A) or an anti-tubulin mAb (B). The full-length and soluble form of CD95L are indicated. Horseradish-peroxidase-labeled goat anti-mouse IgG. Fc Ab was used as the detecting reagent.
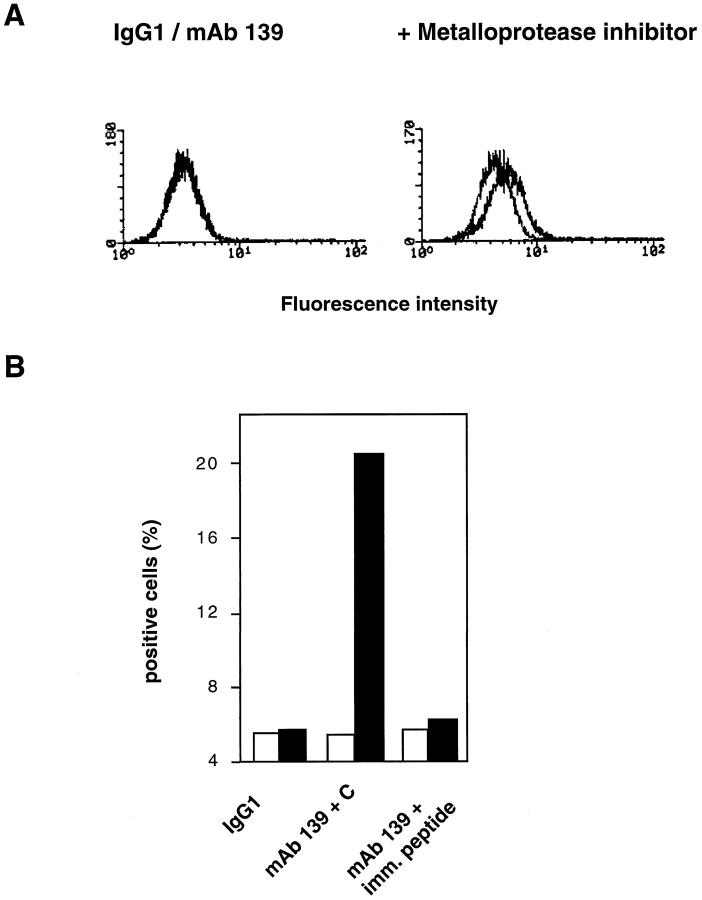
To determine whether mAb 139 reacted with surface-bound CD95L, PMA/ionomycin-activated Jurkat cells were stained with mAb 139 before and after treatment with the metalloprotease inhibitor 1,10-phenanthroline, that induces a two- to fourfold up-regulation of membrane-bound human CD95L. 3 As shown in Figure 3A ▶ , mAb 139 did not detect CD95L on Jurkat cells following stimulation with PMA/ionomycin. However, approximately 20 to 25% of Jurkat cells reacted with mAb 139 following block of CD95L cleavage by the metalloprotease inhibitor. Preincubation of mAb 139 with the immunizing CD95L peptide, but not with an unrelated control (Figure 3B) ▶ , completely blocked binding of mAb 139 to activated Jurkat cells in the presence of 1,10-phenanthroline. Thus, mAb 139 detects the surface-expressed form of human CD95L.
Figure 3.
A: PMA/ionomycin-activated Jurkat cells were stained with mAb 139 before (left panel) or after a 3-hour incubation with the metalloprotease inhibitor 1,10-phenanthroline (right panel) (1.5 mmol/L). Purified mouse IgG1 (background histograms) was used as negative control. Bound mAb was detected with phyco-erythrin-labeled goat anti-mouse IgG. Fc Ab. Results are expressed as histograms of fluorescence intensity. B PMA/ionomycin-activated Jurkat cells were stained with mAb 139 before (white bars) or after a 3-hour incubation with 1,10-phenanthroline (1.5 mmol/L) (black bars ) in the presence of a control unrelated peptide (mAb 139+C) or of the immunizing peptide (mAb 139+imm. peptide). The mouse mAb MOPC21 (IgG1) was used as isotype-matched control. Bound mAb was detected with phyco-erythrin-labeled goat anti-mouse IgG. Fc Ab. Results are expressed as percent positive cells.
Detection of CD95L Transcripts and Protein in Activated T Cells In Vitro and In Vivo
To compare detection of CD95L by in situ hybridization and immunohistochemistry, Jurkat cells were used as reference. Before activation, CD95L transcript signals and antibody binding were minimal. On PMA/ionomycin treatment, however, CD95L mRNA increased significantly and CD95L protein was clearly detectable by mAb G247–4 (Figure 4, A–C) ▶ and mAb 139. We then looked for in vivo-activated cells. In numerous tissues examined, a small subpopulation of intravascular mononuclear cells reacted with our CD95L probe (Figure 4D) ▶ and also with the mAbs (not shown). The same was true for lymphoid cells isolated from inflammatory effusions and from a cystic lymphangioma (Figure 4, E and F) ▶ . Thus, we conclude that our probe and both mAbs are suitable tools for cytological detection of CD95L transcripts and protein, respectively.
Figure 4.
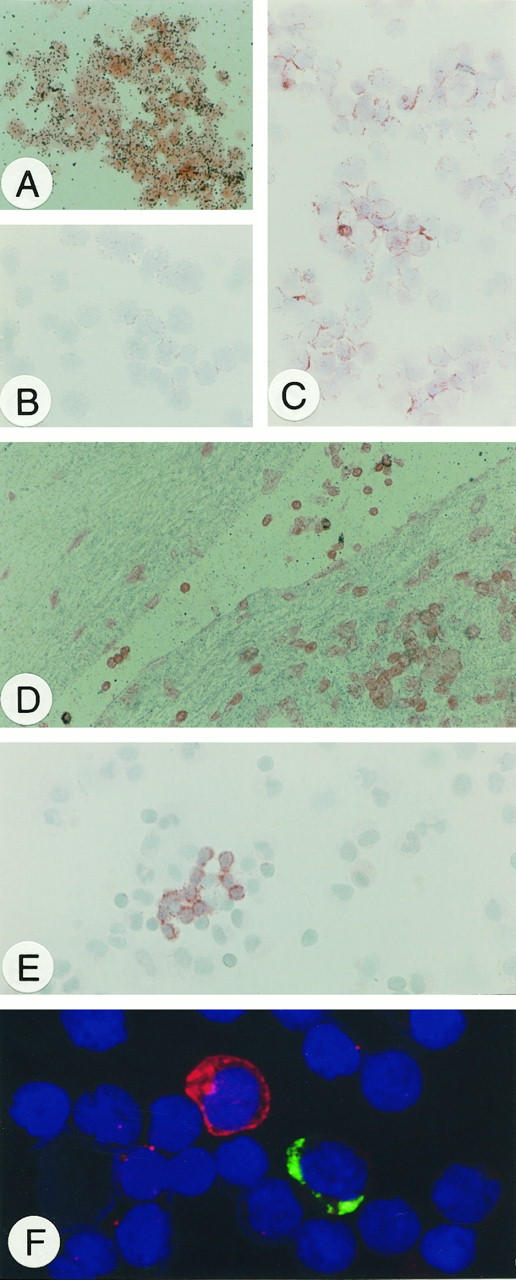
A: Hybridization of CD95L mRNA in PMA/ionomycin-stimulated Jurkat cells. B: Immunohistochemical detection of CD95L protein in unstimulated, C: PMA/ionomycin-treated Jurkat cells. D: In situ hybridization of CD95L mRNA in activated T cells within blood vessels. E: CD95L protein detection by immunohistochemistry in activated T cells in inflammatory pleural effusions. F: Immunofluorescence double staining in lymphoid cells from a cystic lymphangioma. CD95L (red) is detected cytoplasmatically and on the surface of a single lymphoid (probably activated T) cell that lacks IgG expression (green). Original magnification, ×100 (A to E); ×400 (F).
CD95L Detection in Tissue
As shown previously, 26 the CD95L probe worked on paraffin sections with in situ hybridization. The same was true for both anti-CD95L mAbs in immunohistochemistry on frozen sections. However, in formalin fixed material, reaction of both mAbs required pretreatment of tissue sections: mAb G247–4 reacted best after microwave irradiation whereas mAb 139 worked only after pronase digestion (see Materials and Methods). Comparatively, the intensity of the antibody stainings was slightly higher in cryostat sections that, however, displayed a lower morphological resolution (Figure 5A) ▶ . Immunohistology in paraffin sections revealed that, although the pattern of stained cells in most tissues was identical, the mAbs differed in subcellular reactivity: characteristically, G247–4 showed a granular staining pattern concentrated in a distinctive paranuclear area most probably corresponding to the Golgi region (Figure 5B) ▶ . mAb 139 yielded a diffuse staining of the cytoplasm (Figure 5C) ▶ . Cell surface staining was not unequivocally detectable with either of these antibodies, neither in paraffin nor in frozen sections.
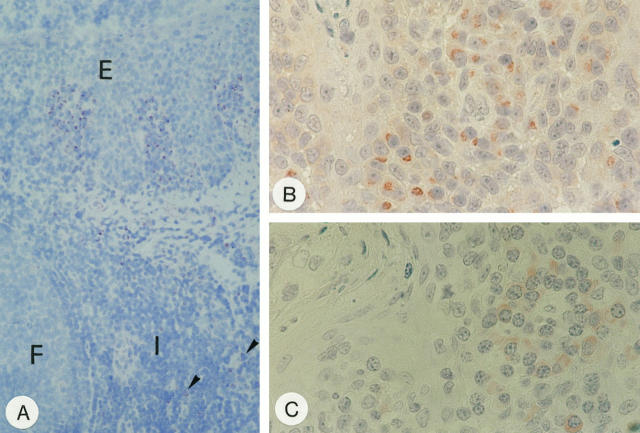
Figure 5.
CD95L protein detection in tissue. A: Immunohistochemistry with mAb G247–4 on frozen section of the tonsil. Note groups of stained cells beneath the squamous epithelium (E) of the crypts with only a few weakly stained cells scattered in the interfollicular areas (I) (arrow heads). F, lymphoid follicle. Immunohistochemistry on paraffin sections using mAb G247–4 (B) and 139 (C). Note different subcellular staining patterns yielded by the two mAbs. In B, nuclear morphology of plasma cells is artificially altered because of microwave irradiation. Plasma cells, however, are readily identified by their broad cytoplasmic rim around an eccentrically located nucleus. Original magnification: ×50 (A); ×157 B and C.
CD95L Expression in Normal Lymphoid Tissues
Thymus
In the thymus, CD95L mRNA was found by in situ hybridization in scattered cells of two distinct zones: 1) the corticomedullary boundary and 2) the subcapsular cortex (Figure 6A) ▶ . CD95L protein was detectable only in scattered lymphoid cells at the corticomedullary boundary in frozen sections with mAb G247–4 (Figure 6B) ▶ , suggesting the presence of very low amounts of CD95L per CD95L+ cell.
Figure 6.
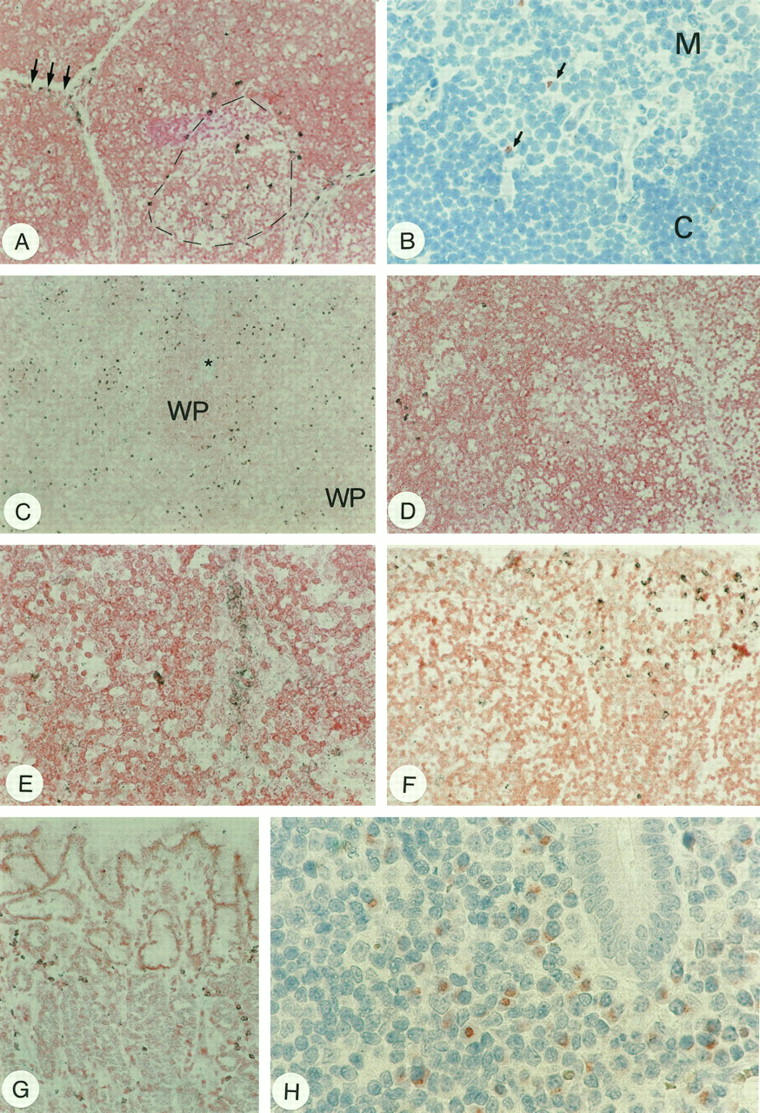
CD95L detection in lymphoid organs. A: In situ hybridization showing CD95L+ cells at the corticomedullary boundary (broken line) and in the subcapsular cortex (arrows) of the thymus. B: Immunohistochemistry reveals weakly positive cells only at the corticomedullary boundary (arrows; M, thymic medulla; C, cortex). C: Detection of CD95L mRNA in the spleen by in situ hybridization. Positive cells are scattered in the red pulp, whereas the white pulp (WP, an arteriole is highlighted by the star) is largely devoid of CD95L+ cells. D: CD95L mRNA-expressing cells in the lymph node were detected by in situ hybridization mainly in the interfollicular areas/medullary cords of the lymph node. E: In some cases, high endothelial venules were labeled. F: In situ hybridization of CD95L mRNA of the tonsil; note strongly labeled cells beneath the surface epithelium. G: Gastric fundus in chronic gastritis of the stomach. Note CD95L+ cells scattered in the lamina propria with in situ hybridization. H shows numerous stained cells in the mucosa of the appendix, most of which are plasma cells. Note again that nuclear morphology of plasma cells is artificially altered by microwave irradiation as in Figure 5B ▶ . Original magnification, ×25 (A, C, D, F, and G); ×100 (B); ×50 (E); ×157 (H).
Spleen
In situ hybridization revealed scattered CD95L mRNA expressing cells confined to the red pulp of normal spleens. The white pulp was essentially negative for CD95L mRNA (Figure 6C) ▶ . This finding was confirmed by immunohistochemistry. Immunoreactivity, however, was rather weak in general. Surprisingly, the stained cells turned out mainly to be plasma cells as revealed by cytomorphology (not shown).
Lymph Node
In the normal, reactive lymph node, very few cells were labeled by in situ hybridization for CD95L mRNA. These CD95L+ cells were mainly scattered in the interfollicular areas and the medullary cords close to the sinuses (Figure 6D) ▶ . No substantial CD95L mRNA expression was observed in follicular mantle and marginal zones. Within germinal centers, especially in hyperplastic ones, a few scattered signals were detected in nonlymphoid cells, presumably histiocytes or dendritic reticulum cells. Inconsistently, epithelioid endothelial cells were found to contain CD95L transcripts (Figure 6E) ▶ . Immunohistochemically, the prevalence of CD95L protein-positive cells was lower. However, the distribution patterns of in situ labeled and immunoreactive cells were very similar. Again, the most prominent producers of CD95L were a subset of plasma cells situated in the medullary cords (not shown, see Figure 5 ▶ ).
Tonsil
In the tonsil, most CD95L mRNA expressing cells were located directly beneath the crypt epithelium (Figure 6F) ▶ and, less frequently, in interfollicular areas. Epithelial cells were negative as were the mantle and marginal zones. Reactivity of germinal centers corresponded to that observed in lymph nodes. Immunohistochemistry revealed a similar, though restricted pattern of CD95L positive cells. Most of these epithelium-associated, CD95L protein-expressing cells were clearly identified as plasma cells by their characteristic morphology (Figure 5, B and C) ▶ .
Stomach and Appendix
In both the appendix and gastric mucosa with acquired mucosa-associated lymphoid tissue (Figure 6G) ▶ , CD95L mRNA expressing cells were scattered within the lamina propria. Immunohistochemistry confirmed this observation. Again, the most prominent subset of CD95L+ cells were identified plasma cells (Figure 6H) ▶ . Furthermore, a small subset of mononuclear CD95L protein-containing lymphoid cells were detected. Interestingly, all intraepithelial lymphocytes lacked detectable CD95L expression (not shown).
CD95L in Tonsillar Plasma Cells Ex Vivo
Morphology in immunohistochemistry was sometimes impaired by previous microwave irradiation in the case of mAb G247–4. To demonstrate unequivocally the plasma cell nature of the strongly labeled cells and possibly to further define the CD95L+ plasma cell population, we performed double immunofluorescence stainings using enriched plasma cell preparations from human tonsils. These stainings clearly showed CD95L expression in cells with a diffuse cytoplasmic positivity for Ig characteristic of plasma cells. Most of the isolated CD95L+ plasma cells were of IgG (Figure 7A) ▶ and IgA (Figure 7B) ▶ phenotype, whereas only very few plasma cells co-expressed IgM (not shown). Thus, CD95L expression is not restricted to but is predominant in IgG and IgA plasma cells.
Figure 7.
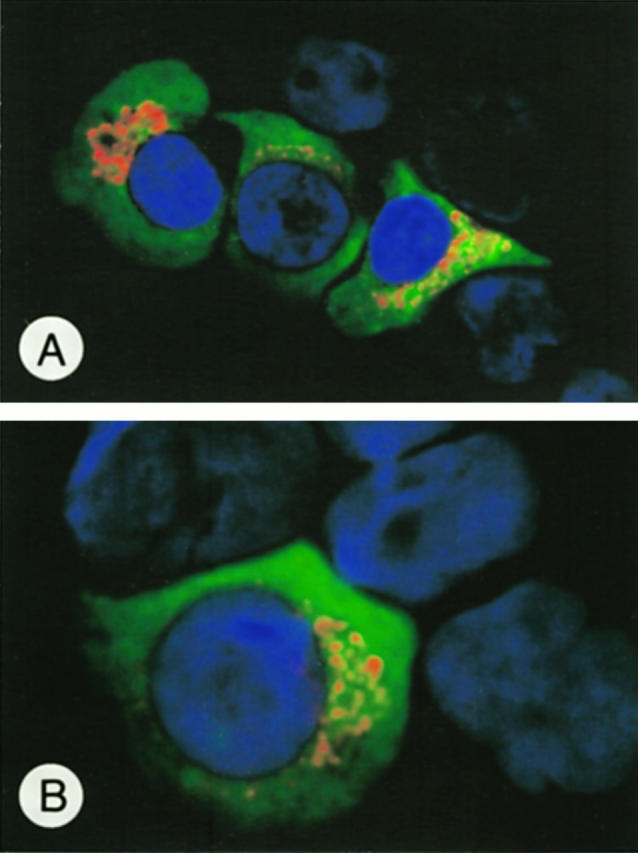
Immunofluorescence double labeling of enriched tonsillar plasma cells. CD95L (red) is expressed in the paranuclear area of cells showing a diffuse cytoplasmic Ig positivity (green) known for plasma cells. Most CD95L+ plasma cells were of IgG (A) or IgA (B) phenotype. Original magnification, ×600 (A); ×1000 (B).
Discussion
T cells, activated by ligation of their T-cell receptor/CD3 complex or by treatment with PMA/ionomycin, rapidly express CD95L and may ultimately undergo CD95-triggered activation-induced cell death (AICD). 5-7 In recent years, functional data on the CD95/CD95L system from in vitro studies have finally given rise to the concept of clonal downsizing of immune responses with the activated T cell as a central player. 17 In fact, in our study, we readily detected CD95L mRNA and protein in in vitro-activated Jurkat cells and in T cells from inflammatory effusions by in situ hybridization and immunostaining. However, using both procedures, CD95L appeared to be expressed in lymphoid tissues in a restricted manner with just a few cells clearly positive for CD95L. Expression levels as reached after PMA/ionomycin treatment of cells in vitro were only rarely found in vivo/in situ. Moreover, in immunohistochemistry, the most prominent fraction of CD95L+ cells in situ were found to be plasma cells, whereas surprisingly, other cells including lymphocytes rarely expressed detectable amounts of CD95L protein. Our inability to detect substantial numbers of CD95L-expressing cells other than plasma cells in immunohistochemistry may, however, reflect a lower level of expression of CD95L in lymphocytes and/or the release of CD95L in its soluble form following proteolytic cleavage. A similar phenomenon is known for other cytokines, most of which cannot be detected immunohistochemically even when present. 29
A critically low level of expression could especially exist in the thymus. In this organ, we found CD95L mRNA by in situ hybridization in cells at the corticomedullary boundary as well as in the subcapsular cortex, although we consistently failed to detect any protein by immunohistochemistry in the subcapsular cortex and detected only weak protein expression in cells of the corticomedullary boundary. Thus, we conclude that 1) in situ hybridization is a more sensitive method to demonstrate CD95L expression than immunohistochemistry and 2) negative results in immunohistochemistry should be interpreted with caution.
Regarding our positive results in normal peripheral lymphoid organs, CD95L mRNA expression was most prominent in areas known to be rich in plasma cells (medullary cords of the lymph node, red pulp of the spleen, and subepithelial areas in the tonsil). Indeed, most of CD95L was, by cytomorphology, attributable to a subset of plasma cells suggesting that these are the main producers of CD95L in the normal peripheral immune system.
CD95L expression was strongest in the mucosa-associated lymphatic tissue of the tonsil and the gastrointestinal tract as demonstrated in the appendix. However, CD95L was not only expressed by plasma cells of the primary, but also of the so-called secondary or acquired mucosa-associated lymphatic tissue, eg, in chronic gastritis. However, in the lymph node and spleen, CD95L expression was restricted to very few immune cells, some of them again plasma cells. Protein concentrations were generally very low. Finally, follicular B cells lacked detectable CD95L expression.
Does our finding of CD95L expression by plasma cells fit into the concept of its role as a immunomodulator? Plasma cells are terminally differentiated B cells releasing soluble antibody. Thus, expression of CD95L might be involved in downsizing the immune response at sites of immunoglobulin secretion. It has to be investigated whether plasma cell-derived CD95L is presented in a membrane-bound manner or secreted into the interstitium. Expression of functionally active CD95L was recently shown on the surface of neoplastic plasma cells, ie, myeloma cells, 30 while we failed to doubtlessly detect a CD95L surface staining by immunohistochemistry. Instead, using mAb G247–4, we found plasma cells to exhibit a paranuclear cap-like staining, a pattern most likely to represent the Golgi compartment. This pattern also suggests that plasma cells may release soluble CD95L.
What could be the possible target for plasma cell-derived CD95L? As untransformed plasma cells lack CD95, 31-33 it is unlikely that they undergo CD95-mediated apoptosis in an autocrine or paracrine way as is known for T cells. 5-7 Thus, CD95L might be directed against locally activated, apoptosis sensitive T cells.
Another cell type strongly expressing CD95 and, thus, a potential target for plasma cell-derived ligand is the tonsillar/gastrointestinal epithelium. 32 Our own studies in ulcerative colitis suggested that CD95L released by lamina propria cells may lead to microlesions of the colonic epithelium through CD95-mediated apoptosis of colonocytes. 27 This, however, is obviously very unlikely in the physiological inflammation in the tonsil or gastrointestinal mucosa. Interestingly, recent studies suggest that soluble CD95L may be less efficient in inducing apoptosis than membrane-bound CD95L and, in circumstances still ill-defined, even block apoptosis mediated by membrane-bound CD95L. 34 Furthermore, anti-CD95 Abs do not kill HT-29 cells, a human colonic carcinoma cell line, but induce interleukin-8 secretion. 35 Thus, it is conceivable that CD95L may have other functional properties in addition to induction of apoptosis. 36
In conclusion, the plasma cell as the final stage of B-cell differentiation may be an as yet underestimated regulator of immune responses/inflammatory processes.
Acknowledgments
The mouse anti-egp34 IgG1 mAb HEA125 was kindly provided by G. Moldenhauer, German Cancer Research Center, Heidelberg, Germany. The authors thank S. Westenfelder, B. Dörr, M. Pach, and S. Welsch for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Jörn Sträter, Institute of Pathology, University of Ulm, Albert-Einstein-Allee 11, D-89081 Ulm, Germany.
Supported by a grant from the Deutsche Forschungsgemeinschaft (D.0687 STR 543/1–1) and the Medical Faculty of the University of Ulm (P332).
Jörn Sträter, Sara M. Mariani, and Henning Walczak contributed equally to this study.
References
- 1.Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75:1169-1178 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S: Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol 1994, 10:1567-1574 [DOI] [PubMed] [Google Scholar]
- 3.Mariani SM, Matiba B, Bäumler C, Krammer PH: Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol 1995, 25:2303-2307 [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Suda T, Takahashi T, Nagata S: Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J 1995, 14:1129-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH: Autocrine T cell suicide mediated by APO-1/(Fas/CD95). Nature 1995, 373:438-441 [DOI] [PubMed] [Google Scholar]
- 6.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR: Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995, 373:441-444 [DOI] [PubMed] [Google Scholar]
- 7.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A: Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995, 373:444-448 [DOI] [PubMed] [Google Scholar]
- 8.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC: A role for CD95 ligand in preventing graft rejection. Nature 1995, 377:630-632 [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 10.Griffith TS, Ferguson TA: The role of FasL-induced apoptosis in immune privilege. Immunol Today 1997, 18:240-244 [DOI] [PubMed] [Google Scholar]
- 11.Green DR, Ware CF: Fas-ligand: privilege and peril. Proc Natl Acad Sci USA 1997, 94:5986-5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Müller C, Tschopp J: Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues characterized by apoptotic cell turnover. J Cell Biol 1996, 133:335-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J: Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 1996, 274:1363-1366 [DOI] [PubMed] [Google Scholar]
- 15.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells: a mechanism of immune evasion? Nat Med 1996, 2:1361-1366 [DOI] [PubMed] [Google Scholar]
- 16.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: a molecular mechanism of tumor immune privilege. Mol Med 1997, 3:294-300 [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch DH, Ramsdell F, Alderson MR: Fas and FasL in the homeostatic regulation of immune responses. Immunol Today 1995, 16:569-574 [DOI] [PubMed] [Google Scholar]
- 18.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 19.van-Parijs L, Abbas AK: Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol 1996, 8:355-361 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice is explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356:314-317 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S: Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994, 76:969-976 [DOI] [PubMed] [Google Scholar]
- 22.Cohen PL, Eisenberg RA: Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 1991, 9:243-269 [DOI] [PubMed] [Google Scholar]
- 23.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 24.Mariani SM, Matiba B, Sparna T, Krammer PH: Expression of biologically active mouse and human CD95/APO-1/Fas ligand in the baculovirus system. J Immunol Methods 1996, 193:63-70 [DOI] [PubMed] [Google Scholar]
- 25.Mariani SM, Matiba B, Armandola EA, Krammer PH: ICE-like proteases/caspases are involved in TRAIL-induced apoptosis of myeloma and leukemia cells. J Cell Biol 1997, 137:221-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Möller P, Walczak H, Riedl S, Sträter J, Krammer PH: Paneth cells express high levels of CD95 ligand transcripts: a unique property among gastrointestinal epithelia. Am J Pathol 1996, 149:9-13 [PMC free article] [PubMed] [Google Scholar]
- 27.Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P: CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 1997, 113:160-167 [DOI] [PubMed] [Google Scholar]
- 28.Mielke B, Möller P: Histomorphologic and immunophenotypic spectrum of primary gastrointestinal B-cell lymphomas. Int J Cancer 1991, 47:334-343 [DOI] [PubMed] [Google Scholar]
- 29.DeLellis RA: In situ hybridization techniques for the analysis of gene expression: applications in tumor pathology. Hum Pathol 1994, 25:580-585 [DOI] [PubMed] [Google Scholar]
- 30.Villunger A, Egle A, Marschitz I, Kos M, Böck G, Ludwig H, Geley S, Kofler R, Greil R: Constitutive expression of Fas (APO-1/CD95) ligand on multiple myeloma cells: a potential mechanism of tumor-induced suppression of immune surveillance. Blood 1997, 90:12-20 [PubMed] [Google Scholar]
- 31.Möller P, Henne C, Leithäuser F, Eichelmann A, Schmidt A, Brüderlein S, Dhein J, Krammer PH: Coregulation of the APO-1 antigen with intercellular adhesion molecule-1 (CD54) in tonsillar B cells and coordinate expression in follicular center B cells and in follicle center and mediastinal B-cell lymphomas. Blood 1993, 81:2067-2075 [PubMed] [Google Scholar]
- 32.Leithäuser F, Dhein J, Mechtersheimer G, Koretz K, Brüderlein S, Henne C, Schmidt A, Debatin K-M, Krammer PH, Möller P: Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfimily, in normal and neoplastic cells. Lab Invest 1993, 69:415-429 [PubMed] [Google Scholar]
- 33.Merville P, Dechanet J, Desmouliere A, Durand I, de Bouteiller O, Garrone P, Banchereau J, Liu YJ: Bcl-2+ tonsillar plasma cells are rescued from apoptosis by bone marrow fibroblasts. J Exp Med 1996, 183:227-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka M, Itai T, Adachi M, Nagata S: Downregulation of Fas ligand by shedding. Nat Med 1998, 4:31-36 [DOI] [PubMed] [Google Scholar]
- 35.Abreu-Martin MT, Vidrich A, Lynch DH, Targan SR: Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J Immunol 1995, 155:4147-41547594569 [Google Scholar]
- 36.Strasser A, O’Connor L: Fas ligand: caught between Scylla and Charybdis. Nat Med 1998, 4:21-22 [DOI] [PubMed] [Google Scholar]