Abstract
The pathology of multiple sclerosis (MS) is characterized by breakdown of the blood-brain barrier (BBB), accompanied by infiltration of macrophages and T lymphocytes into the central nervous system (CNS). The migration of these cells into the CNS parenchyma may be partly regulated by chemokines. The aim of this study was therefore to investigate the cellular localization of the potent monocyte- and T-cell-attracting chemokine monocyte chemoattractant protein (MCP)-1 by immunohistochemistry on postmortem brain tissue from MS and normal control cases. Brain tissue samples of six MS patients and four patients without a history of brain disease were neuropathologically classified according to characteristic (immuno)histochemical staining patterns. Frozen tissue sections of active demyelinating MS lesions, chronic active demyelinating MS lesions, and normal control brain were immunohistochemically stained with a monoclonal antibody directed against MCP-1. In active demyelinating MS lesions as well as in chronic active MS lesions, reactive hypertrophic astrocytes were strongly immunoreactive for MCP-1, whereas perivascular and parenchymal foamy macrophages did not express MCP-1 protein. These results suggest a significant role for the β-chemokine MCP-1, synthesized in vivo by reactive hypertrophic astrocytes, in the recruitment and activation of myelin-degrading macrophages and thereby contributing to the evolution of MS lesions.
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS), characterized by neurological symptoms caused by impaired nerve conduction. Clinical signs of MS are a result of inflammatory lesions in the CNS. Early in lesion development there is breakdown of the blood-brain barrier (BBB), allowing mononuclear cells to enter the CNS. These cells form perivascular cell infiltrates (cuffs) and migrate into the parenchyma of the brain. The monocytes become activated to parenchymal macrophages, which engulf and degrade myelin, and oligodendrocyte death occurs. 1-3 During demyelination, resident microglial cells and astrocytes become activated, astrogliosis occurs, and the lesion becomes less active. Macrophages seem to play a crucial role in the damage to the myelin sheath and/or oligodendrocytes in MS. Macrophages isolated from the CNS of rats suffering from experimental autoimmune encephalomyelitis (EAE) and MS lesioned tissue produce reactive oxygen species (ROS) and nitric oxide (NO), 4,5 and phagocytosis of myelin proteins in the lesion by macrophages indicates ongoing demyelination. 6,7 Elimination of macrophages during EAE leads to a marked suppression of clinical signs of acute EAE and T-cell-line-mediated and chronic relapsing EAE. 8,9
Chemokines (chemotactic cytokines) are believed to be involved in the attraction of myelin-degrading macrophages into MS lesions. 10 Chemokines can mediate the migration of leukocytes into inflammatory sites as well as the activation of effector functions of leukocytes, including production of ROS and exocytosis of degradative enzymes. 11 Four classes of chemokines have been categorized to date based on structural, genetic, and functional considerations: α (C-X-C), β (C-C), γ (C), and δ (C-X3-C). 12 β-Chemokines have been implicated in diseases characterized by monocyte-rich infiltrates, such as atherosclerosis 13 and rheumatoid arthritis 14 and might also participate in the pathogenesis of MS.
Little is known concerning the role of chemokines in the pathogenesis of MS. Elevated levels of β-chemokines have been found in the cerebrospinal fluid (CSF) of MS patients, 16 but to our knowledge, the cellular localization and distribution of chemokines in MS-affected tissue has not been reported. In the present study, we examined the protein expression of monocyte chemoattractant protein (MCP)-1, a β-chemokine that is a very potent monocyte and T cell chemoattractant. 15 Frozen tissue sections with different cellular activities and normal-appearing white matter (NAWM) adjacent to the lesioned tissue were stained with monoclonal anti-MCP-1 antibody. Immunohistochemical staining of brain tissue derived from normal control cases without neuropathological history served as a control.
Materials and Methods
Human Brain Tissue Samples
Human brain tissue was obtained at autopsy (with short postmortem intervals; see Table 1 ▶ ) from six MS patients and four patients without a history of brain disease. The autopsies were performed under the management of the Netherlands Brain Bank, Amsterdam (coordinator Dr. R. Ravid). In all MS cases, multiple tissue samples were taken from lesions located in the brain. Tissue samples from normal control cases were taken from the subcortical white matter or corpus callosum and cerebellum. The clinical diagnosis of MS was confirmed neuropathologically. Brain tissue samples were snap-frozen in liquid nitrogen and stored at −196°C. Hematoxylin and eosin (H&E)-stained sections were prepared from the obtained brain tissue. Tissue samples derived from MS lesions were stained with the neutral lipid marker Oil Red O (ORO) to delineate areas of myelin breakdown and demyelination, with KP1 (CD68) and LCA (CD45) to detect leukocyte infiltration, and with anti-glial fibrillary acidic protein (anti-GFAP) to determine the extent of astrogliosis (see below).
Table 1.
Details of MS and Normal Control Autopsy Brain Tissue
| Case | Age (years) | Sex | Postmortem delay | Cause of death/neuropathology | |
|---|---|---|---|---|---|
| MS cases | S59 | 34 | F | 6 hours, 50 minutes | Dehydration/definitive MS |
| S116 | 35 | F | 5 hours, 45 minutes | Cachexia/definitive MS | |
| S136 | 46 | M | 8 hours | Cachexia/definitive MS | |
| S232 | 40 | F | 7 hours | Not known/definitive MS | |
| S276 | 56 | M | 5 hours, 30 minutes | Respiratory insufficiency/definitive MS | |
| S283 | 46 | M | 3 hours, 45 minutes | Pneumonia/definitive MS | |
| Normal Control cases | |||||
| S49 | 86 | F | 6 hours | Cachexia | |
| S132 | 77 | F | 6 hours | Respiratory insufficiency | |
| S202 | 75 | M | 7 hours | Myocardial infarction | |
| S281 | 89 | F | 6 hours | Aspiration pneumonia |
F, female, M, male.
Immunohistochemistry
Antibodies used in the present study were mouse anti-human MCP-1 (IgG1), kindly provided by Dr. A. Mantovani, Milano, Italy, 17 mouse anti-human KP1 (CD68; IgG1), mouse anti-human leukocyte common antigen (LCA, CD45; IgG1), and rabbit anti-cow GFAP (DAKO, Copenhagen, Denmark). Purified mouse myeloma protein IgG1 (κ), used as an isotype-specific control antibody, was obtained from ICN Pharmaceuticals (Aurora, OH).
Frozen sections (5 μm thick) of MS lesions and normal control CNS tissue were mounted on poly-l-lysine (PLL)-coated glass slides, air dried, and fixed in acetone for 10 minutes at room temperature (RT). All washes were carried out for 15 minutes with 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4), and antibodies were diluted in PBS containing 1% bovine serum albumin (BSA). To prevent nonspecific binding, sections were preincubated with 10% normal swine serum (for polyclonal antibodies (PAbs)) or with 2% normal rabbit serum (for monoclonal antibodies (MAbs) for 10 minutes at RT. Primary antibodies were diluted in PBS/BSA as follows: MCP-1, diluted 1:100, KP1, 1:400; LCA, 1:50; and GFAP, 1:1000, and incubated for 1 hour at RT, followed by washing. Control sections were incubated with mouse purified IgG1 (1:100 dilution). After washing, immunolabeling with primary antibodies was detected with biotinylated rabbit anti-mouse or biotinylated swine anti-rabbit (DAKO) for 30 minutes at RT and avidin-biotin-peroxidase complexes (ABC, Vector Laboratories, Burlingame, CA) for 1 hour at RT. Peroxidase activity was demonstrated with 0.5 mg/ml 3,3′-diaminobenzidine tetrachloride (DAB; Sigma) in 0.05 mol/L Tris/HCL buffer (pH 7.6) containing 0.03% H2O2. Sections were counterstained with hematoxylin, dehydrated, and mounted in Entellan (Merck, Darmstadt, Germany).
All sections were evaluated by light microscopy, and the MCP-1 immunoreactivity was scored by assignment of −, +, ++, or +++ for increasing immunoreactivity.
Results
Neuropathological Evaluation
Frozen sections from all MS lesions were histochemically stained with H&E and ORO and immunohistochemically with KP1, LCA, and GFAP antibodies to evaluate MS lesion activity. Eight MS lesions contained abundant phagocytic, ORO- and KP1-positive foamy macrophages (Figure 1, A–C) ▶ and were classified as active demyelinating (cases S59, S116, S136, and S232, two lesions with comparable cellular activity derived from each case). Four MS lesions had a hypocellular center containing few KP1-positive macrophages (Figure 1, D and E) ▶ , hypercellular rims containing abundant phagocytic ORO and KP1 immunoreactive foamy macrophages, and reactive astrocytes that immunostained strongly for GFAP (Figure 1F) ▶ and were classified as chronic active demyelinating (cases S276 and S283), confirming the classification previously described. 5,18 In normal control brain, no activity of inflammatory cells was detected.
Figure 1.

Cryostat sections from active demyelinating MS lesions (case S59/S116) and chronic active demyelinating MS lesions (case S276/S283). A: Histochemical staining with the neutral lipid marker Oil Red O (ORO) in an active demyelinating lesion. Abundant lipid-filled ORO-positive perivascular and parenchymal macrophages are distributed throughout the demyelinated lesion (arrowheads). B: Immunohistochemical staining with the macrophage marker KP-1 (CD68) in an active demyelinating MS lesion. Strong immunoreactivity for KP1 was detected in both foamy perivascular and parenchymal macrophages (arrowheads). C: A higher magnification shows the CD68-immunoreactive macrophages (arrowheads). D: Immunohistochemical staining with KP1 MAb in a chronic active demyelinated MS lesion. Fewer KP-1-positive parenchymal macrophages were detected in the lesion center, whereas in the hypercellular rim abundant KP1 immunoreactivity was present in macrophages (arrowheads). LC, lesion center; HR, hypercellular rim. E: ORO staining of a chronic active demyelinating lesion. Degraded lipid was detected in the parenchymal foamy macrophages in the hypercellular rim (arrowheads). F: Immunohistochemical staining with a GFAP-specific PAb. Hypertrophic, reactive astrocytes were detected in the lesion center and the hypercellular rim surrounding the demyelinated region (arrowheads). Magnification, ×100 (B and D), ×200 (A, E, and F), and ×400 (C).
Immunohistochemical Staining for MCP-1 on Brain Tissue Sections
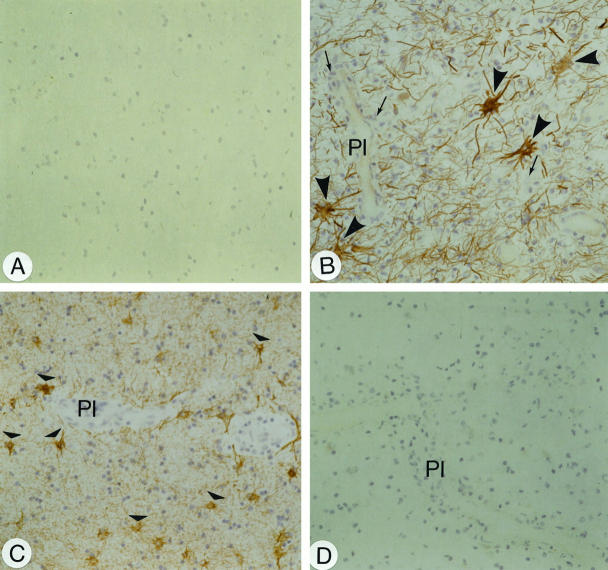
In white matter frozen tissue sections derived from normal control cases, no immunoreactive cells for MCP-1 could be detected (Figure 2A) ▶ .
Figure 2.
Cryostat sections from a normal control case (S49) and an active demyelinating MS lesion (case S116). A: Immunohistochemical staining with a MCP-1 MAb on white matter brain tissue from a normal control case. No MCP-1-immunoreactive cells were found. B: Immunohistochemical staining with a MCP-1 MAb of an active demyelinating MS lesion. Strong to intense immunoreactivity for MCP-1 was detected in virtually all reactive astrocytes (arrowheads) in the surrounding tissue of a perivascular infiltrate located within the demyelinated region. PI, perivascular infiltrate. In perivascular and parenchymal macrophages (arrows) no immunoreactivity for MCP-1 was found. C: Immunohistochemical staining with the astrocytic marker GFAP. Immunoreactivity for GFAP was detected in reactive astrocytes (arrowheads) equally distributed throughout the lesion site. D: No specific immunoreactivity was found in an active demyelinating MS lesion using an isotype-specific control MAb (IgG1). Magnification, ×200.
However, a strong immunoreactivity for MCP-1 MAb was detected in active demyelinating MS lesions in virtually all cells, as could be judged by light microscopy, morphologically resembling reactive astrocytes. Active demyelinating lesions derived from case S116 stained very intensely for the MCP-1 MAb. These particular lesions contained the largest number of active demyelinating macrophages compared with other MS lesions that were investigated for the presence of MCP-1-immunoreactive cells. The MCP-1-immunoreactive cells were distributed throughout the demyelinated region. The MCP-1 protein was detected both in the cell body and in the cell processes (Figure 2B) ▶ . Staining on serial sections using anti-GFAP PAb confirmed the astrocytic phenotype of these cells (Figure 2C) ▶ . Neither macrophages nor lymphocytes present in perivascular infiltrates nor parenchymal (foamy) macrophages in active demyelinating MS lesions showed immunoreactivity for MCP-1 (Figure 2B) ▶ . Using an isotype-specific control MAb, no aspecific staining was detected (Figure 2D) ▶ . In the normal-appearing white matter (NAWM) adjacent to the demyelinated MS lesions, no astrocytes or microglial cells were detected that were immunoreactive for MCP-1 (not shown). The pattern of MCP-1 immunohistochemical staining intensity in normal control and MS cases is summarized in Table 2 ▶ .
Table 2.
Immunohistochemical Staining Pattern of MCP-1 Expression in Normal Control Cases and MS Cases
| Case | MCP-1 in reactive astrocytes |
|---|---|
| Normal control cases | |
| S49 | − |
| S132 | − |
| S202 | − |
| S281 | − |
| Normal-appearing white matter | |
| S116 | − |
| S276 | − |
| Active demyelinating | |
| S59 | ++ |
| S116 | +++ |
| S136 | ++ |
| S232 | ++ |
| Chronic active demyelinating LE/HR | |
| S276 | +/++ |
| S283 | +/++ |
Two lesions per case were examined.
LE, lesion edge; HR, hypercellular rim; −, no staining; +, moderate staining; ++, strong staining; +++, intense staining.
In chronic active demyelinating lesions, cells morphologically resembling reactive astrocytes that were localized in the hypercellular rim of the demyelinated region were strongly immunoreactive for MCP-1 (Figure 3, A ▶ and B). The hypercellular rim contained a larger number of foamy macrophages with ORO-stained degraded lipids, whereas in the lesion center a few KP-1-immunoreactive macrophages with some residual ORO were present (Figure 1, D and E) ▶ . MCP-1 immunoreactivity was predominantly found in the cell processes of the astrocytes. Immunoreactivity became weaker with distance away from the inner edge of the hypercellular rim (Figure 3A) ▶ . At the outer edge of the hypercellular rim, adjacent to the NAWM, the immunoreactivity for MCP-1 in reactive astrocytes was less strong as compared with the strongly MCP-1-immunoreactive cells at the inner edge of the hypercellular rim. Immunohistochemical staining for GFAP on serial sections confirmed their astrocytic phenotype (Figure 3C) ▶ . In the NAWM, no MCP-1-immunoreactive cells were observed (Figure 3D) ▶ , and also in the lesion center no immunoreactivity for MCP-1 was found in the reactive astrocytes that filled up the demyelinated region. Overall, the investigated chronic active MS lesions showed comparable staining patterns for MCP-1 (Table 2) ▶ .
Figure 3.

Cryostat sections from a chronic active demyelinating MS lesion (case S276). A: Immunohistochemical staining with a MCP-1-specific MAb. Reactive astrocytes (arrowheads) stain positive for MCP-1. The immunoreactivity for MCP-1 in the reactive astrocytes became stronger with distance from the lesion center. LC, lesion center; HR, hypercellular rim. B: A reactive astrocyte (arrowhead) at the inner edge of the hypercellular rim of the lesion is strongly immunoreactive for MCP-1. C: Immunohistochemical staining with a GFAP-specific PAb on serial sections. Hypertrophic, reactive astrocytes (arrowheads) were detected in the lesion center and the hypercellular rim surrounding the demyelinated region (arrowheads). D: In the normal-appearing white matter (NAWM) adjacent to the hypercellular rim no immunoreactivity for MCP-1 was detected. Magnification, ×100 (A and B) and ×200 (B and D).
Discussion
Activated inflammatory cells, including macrophages, are thought to be responsible for the breakdown of the myelin sheath, the hallmark of the pathology of MS. 19 Previous reports supported a role for monocyte-attracting and -activating members of the chemokine family in EAE, the animal model for MS. 20,21 In the present study we made the novel observation that a member of the β-chemokines, MCP-1, is abundantly expressed in tissue samples obtained from MS brain with different lesional activity, whereas MCP-1 protein expression is absent in normal white matter CNS tissue. We found strong MCP-1 immunoreactivity in cells that phenotypically were identified as GFAP-immunoreactive astrocytes localized in active demyelinating MS lesions. Interestingly, in the center of chronic active demyelinating MS lesions containing reactive, hypertrophic astrocytes, MCP-1 immunoreactivity was observed in these cells. In this particular site of the lesion, only a few macrophages with residual ORO were present. In these chronic active lesions, a gradient of MCP-1 immunoreactivity was formed beginning at the inner edge of the hypercellular rim surrounding the hypocellular center. In contrast to the lesion center, at the hypercellular rim a large number of ORO-positive macrophages were present, confirming the chemotactic capacity of MCP-1. The absence of MCP-1 immunoreactivity in the center of chronic active demyelinating MS lesions may be caused by shifts in cytokine production, resulting in down-regulation of inflammation. In chronic stages of lesion formation, immunoregulatory cytokines, such as transforming growth factor (TGF)-β, interleukin (IL)-10, and interferon (IFN)-β, that are reported to be immunosuppressive, might be involved. 22
In addition to the recruitment of leukocytes into a site of tissue damage, β-chemokines are involved in the activation of effector functions of leukocytes. MCP-1 stimulates release of lysosomal enzymes and the respiratory burst in monocytes. 11 It is also thought that β-chemokines provide a necessary signal for T lymphocyte activation, 23 thereby facilitating antigen presentation. Furthermore, β-chemokines up-regulate the secretion of the matrix metalloproteinase MMP-9 (92-kd gelatinase) by T lymphocytes. 24 MMPs have the potential to degrade basement membrane and other matrix components, allowing migration of inflammatory cells into the tissue. Recently, we have found expression of MMP-7 on the protein and mRNA level in perivascular and parenchymal macrophages in active demyelinating MS lesions. 25 Blood vessels and mononuclear cells present inside the blood vessels displayed immunoreactivity for MMP-9 in these active lesions. Stüve et al 26 found that chemokines enhanced the production of MMP-9 by peripheral blood cells and that IFN-β1b reduced this MMP-9 production. This is partly accountable for the beneficial effect of IFN-β1b in the treatment of MS patients. In addition to attraction and activation of inflammatory cells, MCP-1 can also activate resident microglial cells and T lymphocytes in MS lesions, which will result in tissue damage and demyelination.
The etiology of MS remains unclear. The interaction of multiple factors likely plays a role in this disease. However, the presence of MCP-1 in the lesions, a mediator with potent inflammation-enhancing properties, such as attraction of monocytes and T cells, induction of enzymes that may contribute to breakdown of the BBB, and activation of monocyte and of T cell effector functions, argues for a participation of β-chemokines in the pathogenesis of MS.
In summary, our results showed protein expression of MCP-1 in active demyelinating lesions and in chronic active MS lesions by reactive hypertrophic astrocytes. Our results suggest a crucial role for β-chemokines in the pathogenesis of both the inflammatory and demyelinating effects occurring in MS lesions. Inhibition of the synthesis of β-chemokines by reactive astrocytes might prove to be beneficial for the treatment of MS in the future.
Acknowledgments
We thank the Netherlands Brain Bank for supplying the human CNS tissue (coordinator, Dr. R. Ravid) and Dr. W. Kamphorst for the neuropathological evaluation. We are also grateful to Jaap van Veldhuisen and Hans Oskam for preparing the illustrations.
Footnotes
Address reprint requests to Dr. Corline J.A. De Groot, Graduate School Neurosciences Amsterdam, Department of Pathology, Division of Neuropathology, Academic Hospital Vrije Universiteit, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands. E-mail: cja.degroot@azvu.nl.
Supported by a grant from the Dutch Foundation Vrienden MS Research.
References
- 1.McDonald WI, Miller DH, Barnes D: The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol 1992, 18:319-334 [DOI] [PubMed] [Google Scholar]
- 2.Constant CF: The pathogenesis of multiple sclerosis. Lancet 1994, 343:271-275 [DOI] [PubMed] [Google Scholar]
- 3.Lassmann H: Basic mechanisms of brain inflammation. J Neural Transm 1997, 50:183-190 [DOI] [PubMed] [Google Scholar]
- 4.Ruuls SR, Bauer J, Sontrop K, Huitinga I, ’t Hart BA, Dijkstra CD: Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J Neuroimmunol 1995, 56:207-217 [DOI] [PubMed] [Google Scholar]
- 5.De Groot CJA, Ruuls SR, Theeuwes JWM, Dijkstra CD, Van Der Valk P: Immunocytochemical characterization of the expression of inducible and constitutive isoforms of nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol 1997, 56:10-20 [DOI] [PubMed] [Google Scholar]
- 6.Bauer J, Sminia T, Wouterlood FG, Dijkstra CD: Phagocytic activity of macrophages and microglial cells during acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res 1994, 38:365-375 [DOI] [PubMed] [Google Scholar]
- 7.Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H: Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995, 38:788-796 [DOI] [PubMed] [Google Scholar]
- 8.Huitinga I, Van Rooijen N, De Groot CJA, Uitdehaag B, Dijkstra CD: Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med 1990, 172:1025-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD: Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental allergic encephalomyelitis in Lewis rats. Clin Exp Immunol 1995, 100:344-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tani M, Ransohoff RM: Do chemokines mediate inflammatory cell invasion of the central nervous system parenchyma? Brain Pathol 1994, 4:135-143 [DOI] [PubMed] [Google Scholar]
- 11.Furie MB, Randolph GJ: Chemokines and tissue injury. Am J Pathol 1995, 146:1287-1301 [PMC free article] [PubMed] [Google Scholar]
- 12.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 13.Takeya M: Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol 1993, 24:534-539 [DOI] [PubMed] [Google Scholar]
- 14.Strieter RM, Koch AE, Antony VB, Fick RB, Jr, Strandiford TJ, Kunkel SL: The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med 1994, 123:183-197 [PubMed] [Google Scholar]
- 15.Cai JP, Hudson S, Ye MW, Chin YH: The intracellular signalling pathways involved in MCP-1-stimulated T cell migration across microvascular endothelium. Cell Immunol 1996, 167:269-275 [DOI] [PubMed] [Google Scholar]
- 16.Myagishi R, Kikuchi S, Fukazawa T, Tashiro K: Macrophage inflammatory protein-1α in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological diseases. Neurol Sci 1995, 129:223-227 [DOI] [PubMed] [Google Scholar]
- 17.Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sozzani S, Coletta I, Mantovani A: A new monoclonal antibody (5D3–F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines: development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods 1994, 174:249-257 [DOI] [PubMed] [Google Scholar]
- 18.Bö L, Mork S, Kong PA, Nyland H, Pardo CA, Trapp BD: Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol 1994, 51:135-146 [DOI] [PubMed] [Google Scholar]
- 19.Lassmann H: Comparative Neuropathology of Chronic Experimental Encephalomyelitis and Multiple Sclerosis. 1983. Springer-Verlag, Berlin [PubMed]
- 20.Myagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K: Identification of cell types producing RANTES, MIP-1α and MIP-1β in rat experimental autoimmune encephalomyelitis by in situ hybridization. J Neuroimmunol 1997, 77:17-26 [DOI] [PubMed] [Google Scholar]
- 21.Godiska R, Chantry D, Dietsch GN, Gray PW: Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol 1995, 58:167-176 [DOI] [PubMed] [Google Scholar]
- 22.Hohlfeld R: Biotechnological agents for the immunotherapy of multiple sclerosis. Brain 1997, 120:865-916 [DOI] [PubMed] [Google Scholar]
- 23.Taub D, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ: Chemokines and T lymphocyte activation. β-chemokines co-stimulate human T lymphocyte activation. J Immunol 1996, 156:2095-3000 [PubMed] [Google Scholar]
- 24.Johnatty RN, Taub DD, Reeder SP, Turcovski-Corrales SM, Cottam DW, Stephenson TJ, Rees RC: Cytokine and chemokine regulation of pro-MMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol 1997, 158:2327-2333 [PubMed] [Google Scholar]
- 25.Cossins J, Clements J, Ford J, Miller KM, Pigott R, Vos W, Van Der Valk P, De Groot CJA: Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol 1997, 94:590-598 [DOI] [PubMed] [Google Scholar]
- 26.Stüve O, Chabot S, Jung SS, Williams G, Yong VW: Chemokine-enhanced migration of human peripheral blood mononuclear cells is antagonized by interferon beta-1b through an effect on matrix metalloproteinase-9. J Neuroimmunol 1997, 80:38-46 [DOI] [PubMed] [Google Scholar]