Abundant intraneuronal neurofibrillary lesions within certain brain regions constitute a defining neuropathological characteristic of Alzheimer’s disease. 1 Ultrastructurally, the neurofibrillary lesions consist of abnormal filamentous deposits in the form of paired helical filaments (PHFs) and the related straight filaments (SFs). These filaments are made of the microtubule-associated protein tau in a hyperphosphorylated state. In normal brain, tau protein is soluble and nonfilamentous. Its ordered assembly into filaments is therefore a pathological event. Tau pathology is not limited to Alzheimer’s disease but is also present in a number of other dementing disorders, such as Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration. 2,3 In these disorders, as in Alzheimer’s disease, the hyperphosphorylated tau protein is filamentous. However, the filament morphologies and tau isoform compositions differ from those of Alzheimer’s disease. The good correlation between the presence of tau pathology and the degree of cognitive impairment has suggested that the events leading to the formation of tau filaments or the mere presence of these filaments are sufficient to produce nerve cell degeneration. Recently, this view has been significantly reinforced by the discovery of mutations in the tau gene in familial frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). 4-8 The new work will no doubt lead to increased efforts aimed at producing experimental animal models of the tau pathology of Alzheimer’s disease and other tauopathies.
Tau Protein and its Assembly into Filaments
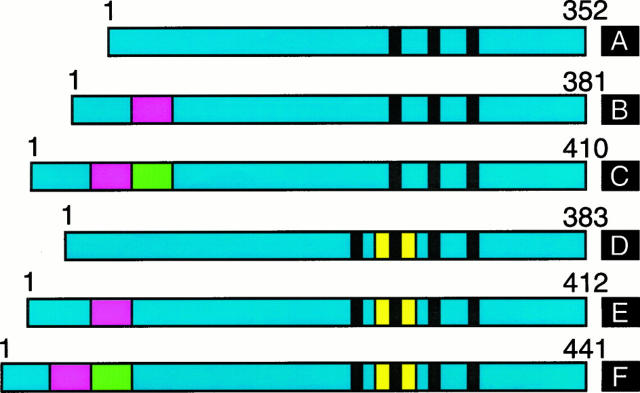
Tau protein promotes microtubule assembly and binds to microtubules, which are thus stabilized. In adult human brain six tau isoforms are expressed; they are produced by alternative mRNA splicing from a single gene located on the long arm of chromosome 17 (Figure 1) ▶ . They differ by the presence of three or four tandem repeats of 31 or 32 amino acids each located in the carboxyl-terminal region in conjunction with 0, 29, or 58 amino acid inserts located in the amino-terminal region. 9,10 There is also a larger tau isoform, with an additional insert in the amino-terminal region, which is mainly expressed in the peripheral nervous system. 11,12 Eleven exons contribute to the longest human brain tau isoform, with exons 2, 3, and 10 being subject to alternative mRNA splicing. 9,10,13 Tau expression is developmentally regulated in that only the tau isoform with three repeats and no amino-terminal inserts is present in fetal brain. There exist true species differences in the expression of tau isoforms in adult brain. Thus, only four-repeat tau isoforms are expressed in rodent brain. By contrast, all six tau isoforms are expressed in adult human brain, where tau isoforms with three repeats are slightly more abundant than tau isoforms with four repeats. The repeat regions of tau and sequences flanking the repeats constitute microtubule-binding domains. 14,15 Tau is expressed predominantly in nerve cells, with lower levels in some glial cells. Within nerve cells, it is found mainly in axons. 16 Inactivation of the tau gene by homologous recombination leads to no overt phenotype, indicating that tau is not an essential protein. 17
Figure 1.
Isoforms of human brain tau. The region common to all isoforms is shown in blue, with the amino-terminal inserts encoded by exons 2 and 3 shown in red and green, respectively. The alternatively spliced repeat encoded by exon 10 is in yellow. The three or four tandem repeats are indicated by black bars. The tau isoforms range from 352 to 441 amino acids in length. Isoform A is expressed in fetal brain, whereas all six isoforms (A-F) are expressed in adult human brain. Transgenic mouse models based on the expression of isoform F 48 or isoform A 49 have been described.
Tau is a phosphoprotein and phosphorylation is also developmentally regulated. Thus, tau from developing brain is phosphorylated more than tau from adult brain. Tau from the PHFs and SFs of Alzheimer’s disease brain is hyperphosphorylated and abnormally phosphorylated on all six isoforms compared to tau from normal adult human brain. 18,19 This contrasts with progressive supranuclear palsy and corticobasal degeneration, where only four-repeat tau isoforms are found in the abnormal filaments. 20-22 In Pick’s disease, the tau filaments consist only of three-repeat isoforms. 23 Hyperphosphorylation and abnormal phosphorylation are major biochemical abnormalities of filamentous tau. They are early events in the development of tau filaments and as a result tau is unable to bind to microtubules. 24-26 However, it is unclear whether hyperphosphorylation and abnormal phosphorylation are sufficient for the assembly of tau into filaments.
Tau Mutations in FTDP-17
Over the past few years, familial frontotemporal dementias, some with parkinsonism, have been recognized as FTDP-17, a previously unknown group of dementia disorders. 27 Their unifying pathological characteristic is the presence of abundant filamentous hyperphosphorylated tau deposits in the absence of Aβ amyloid plaques. In some of these families tau deposits are found in both nerve cells and glial cells, whereas in others only nerve cells are affected. 28
Besides having a filamentous tau pathology in common, the familial frontotemporal dementias also share linkage to chromosome 17q21-22, the same region that contains the tau gene. 29 Recently, the first mutations in the tau gene have been identified in several of these families. 4-8 They are either missense mutations in the microtubule-binding repeat region and the carboxy-terminal region or intronic mutations that change the ratio of three-repeat to four-repeat tau isoforms. Missense mutations have been found in exons 9, 10, 12, and 13 of the tau gene; they change glycine residue 272 to valine (G272V), asparagine residue 279 to lysine (N279K), proline residue 301 to leucine (P301L), valine residue 337 to methionine (V337M), and arginine residue 406 to tryptophan (R406W) (numbering accords with the 441-amino acid isoform of human tau). The N279K and P301L mutations lie in the extra repeat of tau, thus affecting only four-repeat tau isoforms. By contrast, the other three missense mutations are found in all six brain tau isoforms. Four different intronic mutations are found in the region of the exon 10 splice-donor site, where they disrupt a predicted stem-loop. This disruption leads to increased splicing of exon 10, resulting in the overproduction of four-repeat tau isoforms and reduced levels of tau isoforms with three repeats. 5,6
The functional consequences of missense mutations in tau have been studied in microtubule assembly experiments. 30 All the mutations investigated showed a markedly reduced ability to promote microtubule assembly. The P301L mutation produced the largest effect, the R406W mutation the smallest effect, and the G272V and V337M mutations intermediate reductions. This partial loss of function may be the primary effect of these missense mutations in tau. It may be followed by the hyperphosphorylation of tau and, through interaction with other cellular factors, by assembly into filaments. Similarly, overproduction of four-repeat tau isoforms in cases with intronic mutations may result in the inability of some of the excess tau to bind to microtubules, leading to its hyperphosphorylation and assembly into filaments. Most missense mutations are likely to lead to a reduced ability of tau to interact with microtubules. The N279K mutation may be an exception, since it creates an exon splice enhancer sequence, which may lead to increased splicing of exon 10. 8 There may be mutations in tau that produce effects on both microtubule assembly and on mRNA splicing of exon 10.
In Seattle family A (with the V337M mutation), in familial multiple-system tauopathy with presenile dementia (with the +3 mutation in the intron following exon 10), in the Iowa family (with the R406W mutation), in pallido-ponto-nigral degeneration (with the N279K mutation), and in Dutch family 1 (with the P301L mutation), tau is hyperphosphorylated at the same sites as in Alzheimer’s disease. 31-35 Pick-like bodies have been described in Dutch family 2 (with the G272V mutation) that show tau staining characteristics similar to those of classical Pick bodies. 35,36
The balance between tau protein levels and available binding sites on microtubules appears to be critical for determining whether or not tau assembles into filaments. Thus, a reduced ability to interact with microtubules appears to be the shared primary abnormality in tau protein resulting from the different exonic and intronic mutations described thus far. A partial loss of function may be necessary for the assembly of tau into filaments.
The locations of the tau mutations appear to determine the nature of the pathology. Mutations in exon 10 or in the intron following exon 10 lead to a filamentous neuronal and glial cell tau pathology. 32,34,35 For exon 10 mutations, the filaments are narrow twisted ribbons consisting predominantly of tau isoforms with four microtubule-binding repeats. 35 In the case of the intronic mutations, the filaments are wide twisted ribbons consisting exclusively of four-repeat tau isoforms. 32 This is reminiscent of progressive supranuclear palsy and corticobasal degeneration, suggesting that these largely sporadic diseases may also result from abnormalities in the splicing of exon 10 of the tau gene. Missense mutations located outside exon 10 lead to a predominantly neuronal pathology. 31,33 The tau filaments are PHFs and SFs and consist of all six tau isoforms. In the case of the V337M mutation in exon 12, the tau filaments have been shown to be indistinguishable from those of Alzheimer’s disease. 31
Synthetic Tau Filaments
Phosphorylated full-length recombinant tau has consistently failed to assemble into PHF-like filaments in in vitro experiments. By contrast, incubation of recombinant tau with sulphated glycosaminoglycans, such as heparin and heparan sulphate, results in the bulk assembly of tau into Alzheimer-like filaments. 37-42 Tau isoforms with three repeats assemble into twisted paired helical-like filaments, whereas tau isoforms with four repeats assemble into straight filaments. 37
Immunoelectron microscopy shows that the paired helical-like filaments are decorated by antibodies directed against the amino- and carboxy-termini of tau, but not by an antibody directed against the microtubule-binding repeat region. 37 These results, which indicate that in the filaments the repeat region of tau is inaccessible to the antibody, are identical to those previously obtained with PHFs from the brains of Alzheimer’s disease patients. 18 They establish that the microtubule-binding repeat region of tau is essential for sulphated glycosaminoglycan-induced filament formation. Three microtubule-binding repeats of tau are also believed to form the core of the PHFs found in the brains of Alzheimer’s disease patients, supporting the evidence for a similar organization of the two types of filament. Previous experiments had shown that three recombinant microtubule-binding repeats of tau assemble into twisted filaments in vitro. 43,44 This assembly is phosphorylation-independent and occurs in the absence of sulphated glycosaminoglycans. It confirms that three repeats are required to give the morphology of the PHF. However, these experiments do not shed light on the mechanisms that lead to tau filament formation in the brains of Alzheimer’s disease patients, because PHF-tau is made of full-length tau. The dimensions of tau filaments formed in the presence of sulphated glycosaminoglycans are similar to those of filaments extracted from brains of Alzheimer’s disease patients, with diameters of approximately 20 nm for twisted and 15 nm for straight filaments and a crossing-over spacing of approximately 80 nm for paired helical-like filaments, although their twist is in general less regular than that found in Alzheimer’s disease filaments.
Sulphated glycosaminoglycans also stimulate phosphorylation of tau by a number of protein kinases, prevent the binding of tau to taxol-stabilized microtubules, and disassemble microtubules assembled from tau and tubulin. 37,40 Moreover, heparan sulphate has been detected in nerve cells in the early stages of neurofibrillary degeneration. 37,45 Sulphated glycosaminoglycans stimulate tau phosphorylation at lower concentrations than those required for filament formation. The pathological presence of heparan sulphate within the cytoplasm of some nerve cells, perhaps as a result of leakage from membrane-bound compartments, would first lead to hyperphosphorylation of tau, resulting in its inability to bind to microtubules. At higher concentrations of heparan sulphate, tau would then assemble into PHFs and SFs.
Formation of tau filaments is also observed after incubation of recombinant tau with RNA, which has been shown to be sequestered in the neurofibrillary lesions of Alzheimer’s disease. 40,46,47 Whether the presence of RNA is an early event remains to be determined. Sulphated glycosaminoglycans and RNA share a repeat sugar backbone and negative charges in the form of sulphates or phosphates. Tau protein is thought to be an extended molecule with little secondary structure that becomes partially structured upon binding to microtubules. Binding of sulphated glycosaminoglycans or RNA to tau may induce or stabilize a conformation of tau that brings the microtubule-binding repeats of individual tau molecules into close proximity, creating sites which favor polymerization into filaments.
Transgenic Mice
The work on synthetic tau filaments has provided the first robust method for producing Alzheimer-like filaments from full-length tau. The same cannot yet be said of tau filaments in nerve cells. To date, there has been no demonstration of Alzheimer-like filaments in transgenic mice. Two studies have directly addressed this issue by expressing wild-type human tau in the brains of transgenic mice. 48,49 It has been indirectly addressed in the transgenic mouse models of Aβ amyloid deposition, which are based on the expression of mutated amyloid precursor protein (APP). 50-52 Although some staining for hyperphosphorylated tau has been described in nerve cell processes around Aβ deposits in transgenic mice expressing mutated APP, 52,53 no somatodendritic staining of hyperphosphorylated tau was observed in these mice. Two of these mouse lines did not exhibit nerve cell loss, 54,55 whereas a third showed a 17% reduction in the number of nerve cells in layer CA1 of the hippocampus. 56 However, it remains to be seen whether this cell loss is mechanistically related to the nerve cell loss observed in Alzheimer’s disease hippocampus. Mutated APP is expressed at high levels in these mice and this could in itself result in degeneration of some nerve cells. It is well established that in Alzheimer’s disease brain there exists an inverse correlation between the number of extracellular tangles and the number of surviving nerve cells in the hippocampus, 57-59 suggesting that nerve cell loss is due to the formation of neurofibrillary lesions.
The first study expressing human tau protein in transgenic mice was published in 1995 and described the expression of the longest human brain tau isoform (four repeats and the 58-amino acid amino-terminal insert) under the control of the human Thy1 promoter 48 (Figure 1) ▶ . The new study, which describes expression of the shortest human brain tau isoform (three repeats and no amino-terminal inserts) under the control of the mouse 3-hydroxy-methyl-glutaryl CoA reductase promoter, is published in this issue of the Journal 49 (Figure 1) ▶ .
Both studies describe broadly similar results with some minor differences. They show strong somatodendritic and axonal staining for hyperphosphorylated tau of subpopulations of nerve cells. The somatodendritic staining is pathological, because in control mouse brain tau staining is largely axonal. Götz et al described only nerve cell staining, 48 whereas Brion et al also describe some astrocytic staining, 49 presumably reflecting the use of a different promoter. The presence of hyperphosphorylated human tau in mouse brain astrocytes is interesting in view of the extensive glial tau pathology seen in some FTDP-17 pedigrees, as well as in progressive supranuclear palsy and corticobasal degeneration. Both studies show somatodendritic staining of nerve cells with a number of phosphorylation-dependent anti-tau antibodies that also stain the neurofibrillary pathology of Alzheimer’s disease and other tauopathies. These antibodies also recognize tau from normal adult human brain, albeit more weakly than PHF-tau. Brion et al show that antibodies which are entirely specific for PHF-tau, such as AP422 and AT100, 60-62 do not stain transgenic mouse brain, a finding in agreement with the lack of tau filaments. By electron microscopy, they show that transgenic human tau is associated with microtubules in axons and dendrites, but not in nerve cell bodies, where it is associated with ribosomes or distributed more diffusely. 49 Overexpression of human tau in lamprey neurons has also been shown to lead to the presence of hyperphosphorylated human tau in the somatodendritic compartment. 63 It thus appears that an excess of tau over available binding sites on microtubules results in the accumulation of tau in nerve cell bodies. The same may be true of the FTDP-17 cases with intronic mutations in the tau gene.
Somatodendritic staining for hyperphosphorylated tau has been described as an early pathological change in human brain, where it is characteristic of the so-called pre-tangle stage of Alzheimer’s disease. 24 In human brain, the pre-tangle pathology progresses to the tangle stage, which is followed by nerve cell degeneration and death. In the case of the classical neurofibrillary tangle, thick bundles of tau filaments survive the death of affected nerve cells and are found in the extracellular space in the form of ghost tangles. 1 The presence of neurofibrillary tangles does not appear to be a necessary prerequisite for nerve cell degeneration, because they are absent from a number of FTDP-17 brains. 28 The invariant feature of the various tauopathies is the presence of filaments made of hyperphosphorylated tau protein. So far, such filaments have not been observed in the brains of mice transgenic for tau protein. There is no evidence to suggest nerve cell loss in the mice, indicating that the prolonged presence of hyperphosphorylated tau in the somatodendritic compartment of nerve cells is not sufficient to lead to nerve cell degeneration. The current transgenic mouse models thus go only part of the way towards establishing a filamentous tau pathology.
The levels of expression of human tau protein in the transgenic mouse lines were relatively modest, ranging between 10–20% of total mouse brain tau. Adult mouse brain tau consists of three four-repeat isoforms, whereas only one human tau isoform was expressed in each of the transgenic mouse studies. However, human tau appeared to be concentrated in a relatively small number of nerve cells, suggesting that the levels of human tau per cell may be much higher. Nevertheless, the failure to form tau filaments in mouse brain may be due to insufficient levels of human tau. From the experiments on synthetic tau filaments, it is clear that the assembly of recombinant tau in presence of sulphated glycosaminoglycans is strongly concentration-dependent, as befits a nucleation-dependent process. 37-40,42 Other differences between mice and humans may also play a role. Mice express only three four-repeat tau isoforms in adult brain, whereas humans express an additional three isoforms with three repeats. If cellular factors are needed to induce tau filament formation, they may not be present in sufficient concentrations in mouse brain. Finally, differences may be a function of the very different life spans of mouse and human.
Outlook
The discovery of missense mutations in tau in FTDP-17 has demonstrated that tau dysfunction produces neurodegeneration. The existence of mutations in the intron following exon 10 of the tau gene has shown that the simple overproduction of four-repeat tau is sufficient to lead to a filamentous pathology and to produce a dementia disorder. This knowledge will be invaluable for future efforts aimed at producing mouse lines transgenic for tau. Higher expression levels of human transgenic tau than have been achieved so far may be the key to success. Animal models of FTDP-17, Pick’s disease, progressive supranuclear palsy, and corticobasal degeneration are eagerly awaited. Perhaps most importantly, there will be no true transgenic mouse model of Alzheimer’s disease without a filamentous tau pathology.
Footnotes
Address reprint requests to Michel Goedert, Medical Research Council Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK. E-mail: mg@mrc-lmb.cam.ac.uk.
References
- 1.Spillantini MG, Goedert M: Tau protein pathology in neurodegenerative diseases. Trends Neurosci 1998, 21:428-433 [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW: Pick’s disease: a modern approach. Brain Pathol 1998, 8:839-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron C, Davis A, Lang AE: Corticobasal degeneration and progressive supranuclear palsy presenting with cognitive decline. Brain Pathol 1998, 8:355-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD: Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998, 43:815-825 [DOI] [PubMed] [Google Scholar]
- 5.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok DBJ, Schofield PR, Andreadis A, Snowden J, Craufurd A, Neary D, Owen F, Oostra BA, Hardy J, Goate A, Van Swieten J, Mann D, Lynch T, Heutink P: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393:702-705 [DOI] [PubMed] [Google Scholar]
- 6.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B: Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998, 95:7737-7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumanchin C, Camuzat A, Campion D, Verpillat P, Hannequin D, Dubois B, Saugier-Veber P, Martin C, Penet C, Charbonnier F, Agid Y, Frebourg T, Brice A: Segregation of a missense mutation in the microtubule-associated protein tau with familial frontotemporal dementia and parkinsonism. Hum Mol Genet 1998, 7:1825-1829 [DOI] [PubMed] [Google Scholar]
- 8.Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D’Souza I, Lee VMY, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC: Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA 1998, 95:13103-13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA: Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau mRNAs in human brain. EMBO J 1989, 8:393-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 11.Goedert M, Spillantini MG, Crowther RA: Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci USA 1992, 89:1983-1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couchie D, Mavilia C, Georgieff IS, Liem RKH, Shelanski ML, Nunez J: Primary structure of high molecular weight tau present in the peripheral nervous system. Proc Natl Acad Sci USA 1992, 89:4378-4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreadis A, Brown MW, Kosik KS: Structure and novel exons of the human tau gene. Biochemistry 1992, 31:10626-10633 [DOI] [PubMed] [Google Scholar]
- 14.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E: Domains of tau protein and interactions with microtubules. Biochemistry 1994, 33:9511-9522 [DOI] [PubMed] [Google Scholar]
- 15.Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, Feinstein SC: Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell 1997, 8:353-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder LI, Frankfurter A, Rebhun LI: The distribution of tau in the mammalian nervous system. J Cell Biol 1985, 101:1371-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Oshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N: Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 1994, 369:488-491 [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Spillantini MG, Cairns NJ, Crowther RA: Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 1992, 8:159-168 [DOI] [PubMed] [Google Scholar]
- 19.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y: Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 1995, 270:823-829 [DOI] [PubMed] [Google Scholar]
- 20.Flament S, Delacourte A, Verny M, Hauw JJ, Javoy-Agid F: Abnormal tau proteins in progressive supranuclear palsy: similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol 1991, 81:591-596 [DOI] [PubMed] [Google Scholar]
- 21.Delacourte A, Buée L: Normal and pathological tau proteins as factors for microtubule assembly. Int Rev Cytol 1997, 171:167-224 [DOI] [PubMed] [Google Scholar]
- 22.Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu WK, Yen SH, Weidenheim K, Dickson DW: Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol 1994, 145:1496-1508 [PMC free article] [PubMed] [Google Scholar]
- 23.Sergeant N, David JP, Lefranc D, Vermersch P, Wattez A, Delacourte A: Different distribution of phosphorylated tau protein isoforms in Alzheimer’s and Pick’s diseases. FEBS Lett 1997, 412:578-582 [DOI] [PubMed] [Google Scholar]
- 24.Braak E, Braak H, Mandelkow EM: A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol 1994, 87:554-562 [DOI] [PubMed] [Google Scholar]
- 25.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Ihara Y: Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament tau. J Neurochem 1993, 61:1183-1186 [DOI] [PubMed] [Google Scholar]
- 27.Foster NL, Wilhelmsen K, Sima AAF, Jones MZ, D’Amato C, Gilman S, Spillantini MG, Lynch T, Mayeux RP, Gaskell PC, Hulette C, Pericak-Vance MA, Welsh-Bohmer KA, Dickson DW, Heutink P, Kros J, Van Swieten JC, Arwert F, Ghetti B, Murrell J, Lannfelt L, Hutton M, Phelps CH, Snyder DS, Oliver E, Ball MJ, Cummings JL, Miller BL, Katzman R, Reed L, Schelper RL, Lanska DJ, Brun A, Fink JK, Khul DE, Knopman DS, Wszolek Z, Miller CL, Bird TD, Lendon C, Elechi C: Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus statement. Ann Neurol 1997, 41:706-715 [DOI] [PubMed] [Google Scholar]
- 28.Spillantini MG, Bird TD, Ghetti B: Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 1998, 8:387-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygaard TG: Localization of disinhibition-dementia-Parkinsonism-amyotrophy complex to 17q21–22. Am J Hum Genet 1994, 55:1159-1165 [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa M, Smith MJ, Goedert M: Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 1998, 437:207-210 [DOI] [PubMed] [Google Scholar]
- 31.Spillantini MG, Crowther RA, Goedert M: Comparison of the neurofibrillary pathology in Alzheimer’s disease and familial presenile dementia with tangles. Acta Neuropathol 1996, 92:42-48 [DOI] [PubMed] [Google Scholar]
- 32.Spillantini MG, Goedert M, Crowther RA, Murrell J, Farlow MJ, Ghetti B: Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA 1997, 94:4113-4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ: Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol 1997, 42:564-572 [DOI] [PubMed] [Google Scholar]
- 34.Reed LA, Schmidt ML, Wszolek ZK, Balin BJ, Soontornniyomkij V, Lee VMY, Trojanowski JQ, Schelper RL: The neuropathology of a chromosome 17-linked autosomal dominant Parkinsonism and dementia (“pallido-ponto-nigral degeneration”). J Neuropathol Exp Neurol 1998, 57:588-601 [DOI] [PubMed] [Google Scholar]
- 35.Spillantini MG, Crowther RA, Kamphorst W, Heutink P, Van Swieten JC: Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. Am J Pathol 1998, 153:1359-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG: Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol 1996, 92:588-596 [DOI] [PubMed] [Google Scholar]
- 37.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA: Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 1996, 383:550-553 [DOI] [PubMed] [Google Scholar]
- 38.Pérez M, Valpuesta JM, Medina M, Montejo de Garcini E, Avila J: Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. J Neurochem 1996, 67:1183-1190 [DOI] [PubMed] [Google Scholar]
- 39.Arrasate M, Pérez M, Valpuesta JM, Avila J: Role of glycosaminoglycans in determining the helicity of paired helical filaments. Am J Pathol 1997, 151:1115-1122 [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa M, Crowther RA, Jakes R, Goedert M: Alzheimer-like changes in microtubule-associated protein tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J Biol Chem 1997, 272:33118-33124 [DOI] [PubMed] [Google Scholar]
- 41.Qi Z, Zhu X, Goedert M, Fujita DJ, Wang JH: Effect of heparin on phosphorylation site specificity of neuronal Cdc2-like kinase. FEBS Lett 1998, 423:227-230 [DOI] [PubMed] [Google Scholar]
- 42.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E: Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry 1998, 37:10223-10230 [DOI] [PubMed] [Google Scholar]
- 43.Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E: Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol 1992, 118:573-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowther RA, Olesen OF, Jakes R, Goedert M: The microtubule-binding repeats of tau protein assemble into filaments like those found in Alzheimer’s disease. FEBS Lett 1992, 309:199-202 [DOI] [PubMed] [Google Scholar]
- 45.Snow AD, Mar H, Nochlin D, Sekiguchi RT, Kimata K, Koike Y, Wight TN: Early accumulation of heparan sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer’s disease and Down’s syndrome. Am J Pathol 1990, 137:1253-1270 [PMC free article] [PubMed] [Google Scholar]
- 46.Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E: RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett 1996, 339:344-349 [DOI] [PubMed] [Google Scholar]
- 47.Ginsberg SD, Crino PB, Lee VMY, Eberwine JH, Trojanowski JQ: Sequestration of RNA in Alzheimer’s disease neurofibrillary tangles and senile plaques. Ann Neurol 1997, 41:200-209 [DOI] [PubMed] [Google Scholar]
- 48.Götz J, Probst A, Spillantini MG, Schäfer T, Jakes R, Bürki K, Goedert M: Somatodendritic localisation and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 1995, 14:1304-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brion JP, Tremp G, Octave JN: Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer’s disease. Am J Pathol 1999, 154:255-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie R, Guido T, Hagopian S, Johnson-Wood K, Khan I, Lee M, Leibowitz P, Liebergurb I, Little S, Masliah E, McColongue L, Montoya Azvala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J: Development of neuropathology similar to Alzheimer’s disease in transgenic mice overexpressing the 717V→F β-amyloid precursor protein. Nature 1995, 373:523-527 [DOI] [PubMed] [Google Scholar]
- 51.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G: Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 1996, 274:99-102 [DOI] [PubMed] [Google Scholar]
- 52.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B: Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA 1997, 94:13287-13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Games D, Masliah E, Lee M, Johnson-Wood K, Schenk D: Neurodegenerative Alzheimer-like pathology in PDAPP 717V→F transgenic mice. Hyman BT Duyckaerts C Christen Y eds. Connections, Cognition and Alzheimer’s Disease. 1997, :pp 105-119 Springer, Berlin, Heidelberg, [Google Scholar]
- 54.Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT: Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci 1997, 17:7053-7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT: APPSw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol 1997, 56:965-973 [DOI] [PubMed] [Google Scholar]
- 56.Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M: Neuron loss in APP transgenic mice. Nature 1998, 395:755-756 [DOI] [PubMed] [Google Scholar]
- 57.Bondareff W, Mountjoy CQ, Roth M, Hauser DL: Neurofibrillary degeneration and neuronal loss in Alzheimer’s disease. Neurobiol Aging 1989, 10:709-715 [DOI] [PubMed] [Google Scholar]
- 58.Cras P, Smith MA, Richey PL, Siedlak SL, Mulvihill P, Perry G: Extracellular neurofibrillary tangles reflect neuronal loss and provide further evidence of extensive protein cross-linking in Alzheimer disease. Acta Neuropathol 1995, 89:291-295 [DOI] [PubMed] [Google Scholar]
- 59.Fukutani Y, Kobayashi K, Nakamura I, Watanabe K, Isaki K, Cairns NJ: Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett 1995, 200:57-60 [DOI] [PubMed] [Google Scholar]
- 60.Hasegawa M, Jakes R, Crowther RA, Lee VMY, Ihara Y, Goedert M: Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett 1996, 384:25-30 [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann R, Lee VMY, Leight S, Varga I, Otvos L: Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry 1997, 36:8114-8124 [DOI] [PubMed] [Google Scholar]
- 62.Zheng-Fischhöfer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E: Sequential phosphorylation of tau by glycogen synthase kinase-3β and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired helical filament-like conformation. Eur J Biochem 1998, 252:542-552 [DOI] [PubMed] [Google Scholar]
- 63.Hall GF, Yao J, Lee G: Human tau becomes phosphorylated and forms filamentous deposits when overexpressed in lamprey central neurons in situ. Proc Natl Acad Sci USA 1997, 94:4733-4738 [DOI] [PMC free article] [PubMed] [Google Scholar]