Abstract
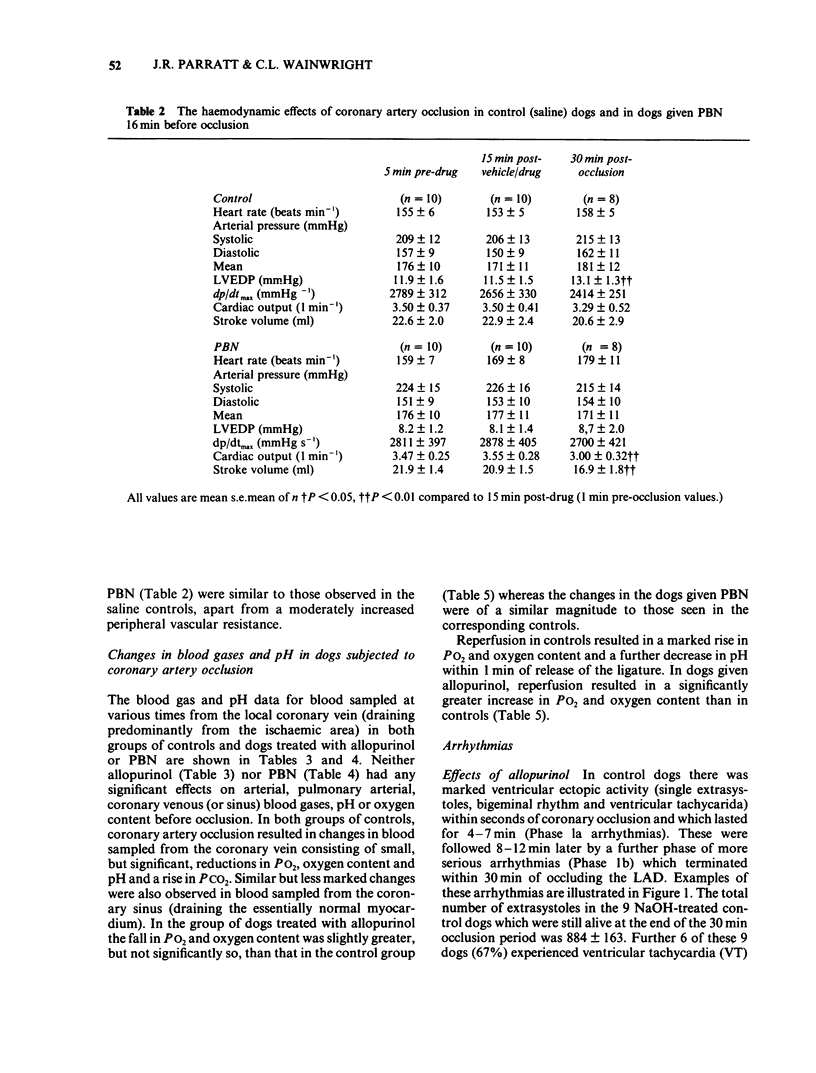
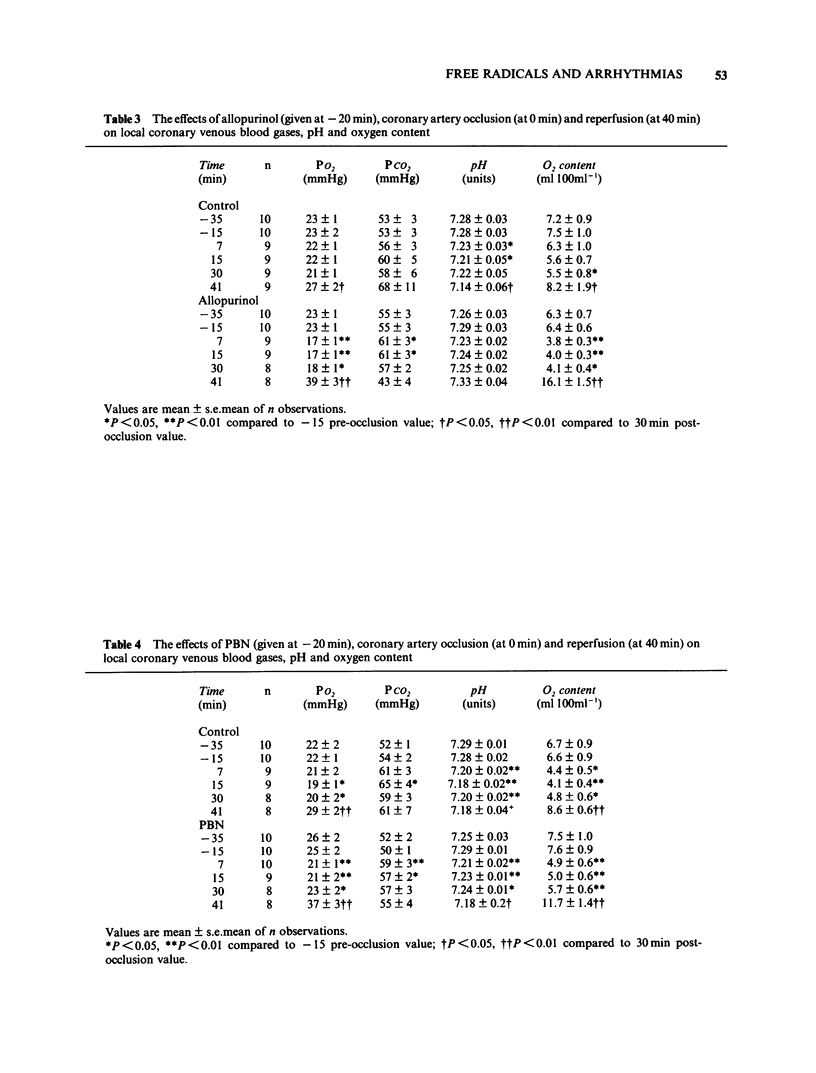
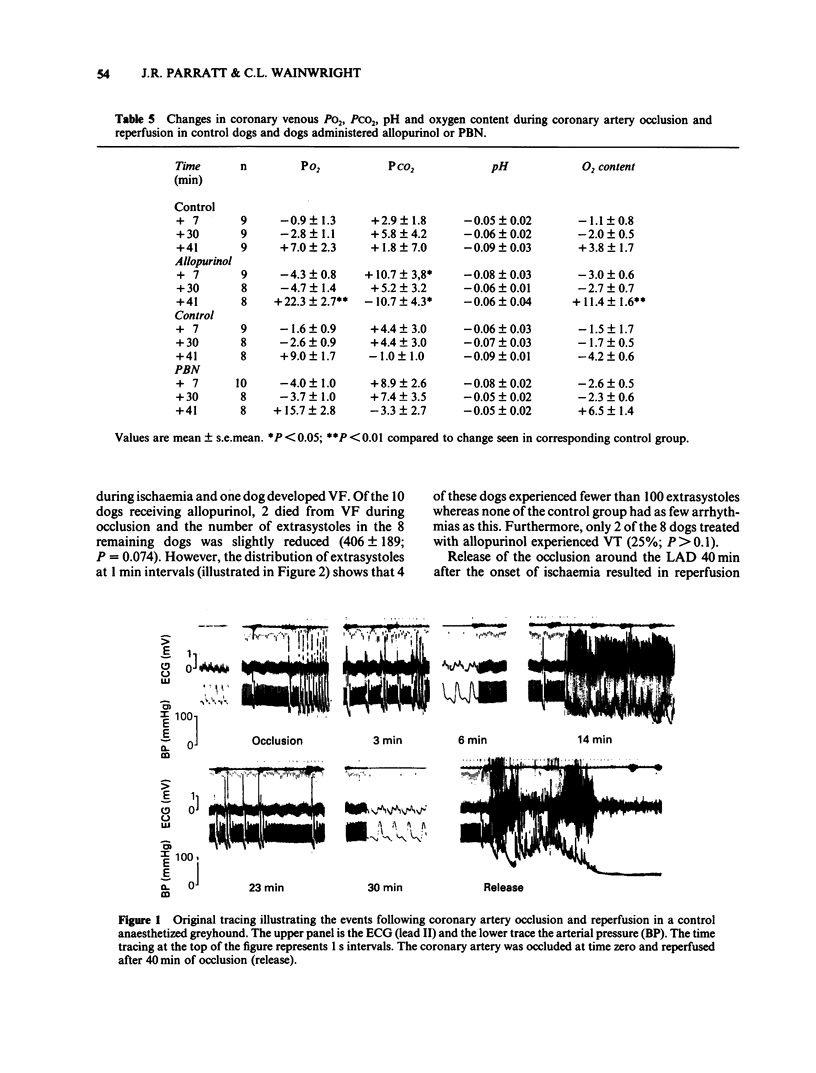
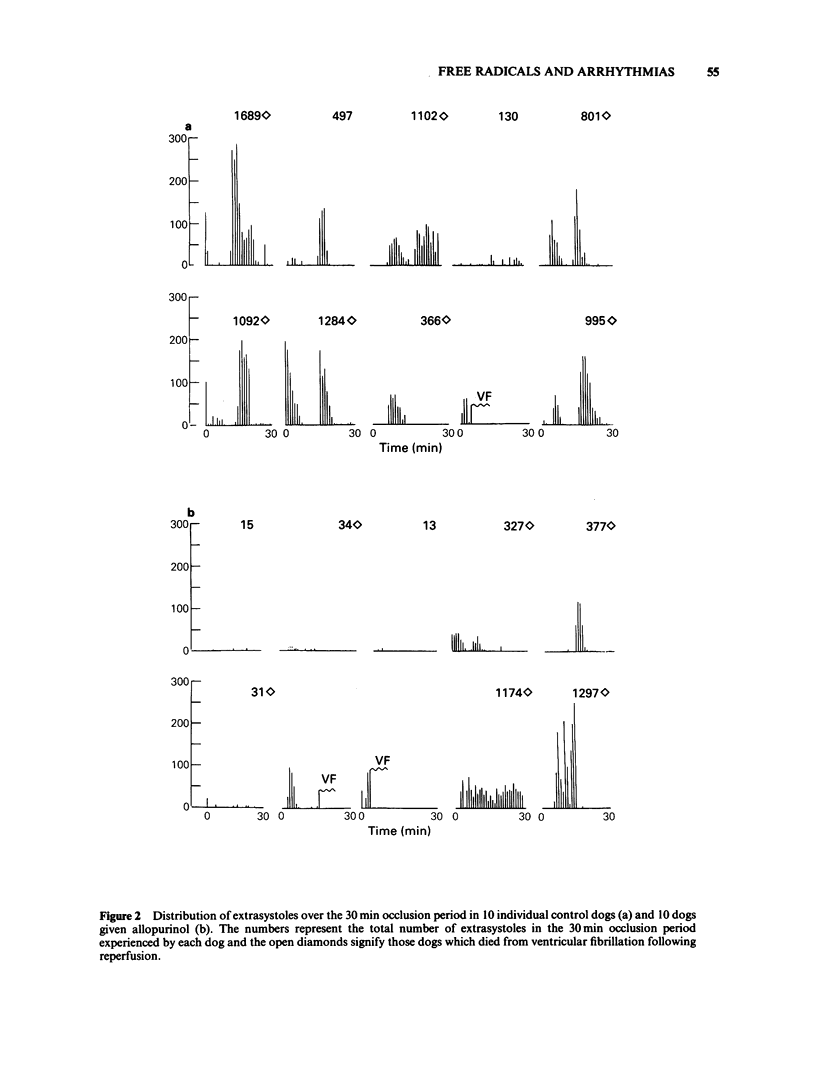
The possible role of free radicals in the genesis of occlusion and reperfusion-induced arrhythmias was studied by determining the effects of the xanthine oxidase inhibitor allopurinol (400 mg p.o. 24 h before experimentation +25 mg kg-1 i.v.) and the free radical scavenger N-t-butyl-alpha-phenyl nitrone (PBN; 50 mg kg-1 i.v.) on these arrhythmias in chloralose anaesthetized greyhounds. Neither of the drugs had any major effects on haemodynamic variables, although allopurinol caused a significant increase in heart rate. The mean number of extrasystoles observed during ischaemia in dogs given allopurinol or PBN was not significantly different from those seen in controls. Further, the incidence of ventricular fibrillation during either occlusion or reperfusion was unchanged by either drug and there was thus no improvement in survival. These results suggest that, in this model of myocardial ischaemia and reperfusion, free radicals may not play a major role in the genesis of life-threatening arrhythmias.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier M., Hearse D. J., Manning A. S. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with "anti-free radical" interventions and a free radical-generating system in the isolated perfused rat heart. Circ Res. 1986 Mar;58(3):331–340. doi: 10.1161/01.res.58.3.331. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974 Apr 25;249(8):2447–2452. [PubMed] [Google Scholar]
- Coker S. J., Parratt J. R., Ledingham I. M., Zeitlin I. J. Evidence that thromboxane contributes to ventricular fibrillation induced by reperfusion of the ischaemic myocardium. J Mol Cell Cardiol. 1982 Aug;14(8):483–485. doi: 10.1016/0022-2828(82)90156-0. [DOI] [PubMed] [Google Scholar]
- Coker S. J., Parratt J. R., Ledingham I. M., Zeitlin I. J. Thromboxane and prostacyclin release from ischaemic myocardium in relation to arrhythmias. Nature. 1981 May 28;291(5813):323–324. doi: 10.1038/291323a0. [DOI] [PubMed] [Google Scholar]
- Gauduel Y., Duvelleroy M. A. Role of oxygen radicals in cardiac injury due to reoxygenation. J Mol Cell Cardiol. 1984 May;16(5):459–470. doi: 10.1016/s0022-2828(84)80617-3. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Farber N. E., Hardman H. F., Warltier D. C. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. Am J Physiol. 1986 Mar;250(3 Pt 2):H372–H377. doi: 10.1152/ajpheart.1986.250.3.H372. [DOI] [PubMed] [Google Scholar]
- Haglund U., Lundgren O. Intestinal ischemia and shock factors. Fed Proc. 1978 Nov;37(13):2729–2733. [PubMed] [Google Scholar]
- Hess M. L., Okabe E., Kontos H. A. Proton and free oxygen radical interaction with the calcium transport system of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1981 Aug;13(8):767–772. doi: 10.1016/0022-2828(81)90258-3. [DOI] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Kubow S., Janzen E. G., Bray T. M. Spin-trapping of free radicals formed during in vitro and in vivo metabolism of 3-methylindole. J Biol Chem. 1984 Apr 10;259(7):4447–4451. [PubMed] [Google Scholar]
- Manning A. S., Coltart D. J., Hearse D. J. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984 Oct;55(4):545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- Marshall R. J., Parratt J. R., Ledingham I. M. Changes in blood flow and oxygen consumption in normal and ischaemic regions of the myocardium following acute coronary artery ligation. Cardiovasc Res. 1974 Mar;8(2):204–215. doi: 10.1093/cvr/8.2.204. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McKechnie K., Furman B. L., Parratt J. R. Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ Shock. 1986;19(4):429–439. [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Myers M. L., Bolli R., Lekich R. F., Hartley C. J., Roberts R. Enhancement of recovery of myocardial function by oxygen free-radical scavengers after reversible regional ischemia. Circulation. 1985 Oct;72(4):915–921. doi: 10.1161/01.cir.72.4.915. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Lai E. K., Janzen E. G., Davis E. R. Confirmation of assignment of the trichloromethyl radical spin adduct detected by spin trapping during 13C-carbon tetrachloride metabolism in vitro and in vivo. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1154–1160. doi: 10.1016/0006-291x(80)90540-9. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Weddle C. C., Downs P. E. In vivo spin-trapping of radicals formed during halothane metabolism. Biochem Pharmacol. 1981 Jun 15;30(12):1517–1519. doi: 10.1016/0006-2952(81)90375-0. [DOI] [PubMed] [Google Scholar]
- RONA G., CHAPPEL C. I., KAHN D. S. THE SIGNIFICANCE OF FACTORS MODIFYING THE DEVELOPMENT OF ISOPROTERENOL-INDUCED MYOCARDIAL NECROSIS. Am Heart J. 1963 Sep;66:389–395. doi: 10.1016/0002-8703(63)90271-0. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Cohen M. V., Mueller H. S. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J Mol Cell Cardiol. 1983 Oct;15(10):713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. Failure of the xanthine oxidase inhibitor allopurinol to limit infarct size after ischemia and reperfusion in dogs. Circulation. 1985 May;71(5):1069–1075. doi: 10.1161/01.cir.71.5.1069. [DOI] [PubMed] [Google Scholar]
- Woodward B., Zakaria M. N. Effect of some free radical scavengers on reperfusion induced arrhythmias in the isolated rat heart. J Mol Cell Cardiol. 1985 May;17(5):485–493. doi: 10.1016/s0022-2828(85)80053-5. [DOI] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Induction of necrosis and failure in the isolated perfused rat heart with oxidized isoproterenol. J Mol Cell Cardiol. 1975 Nov;7(11):807–816. doi: 10.1016/0022-2828(75)90132-7. [DOI] [PubMed] [Google Scholar]



