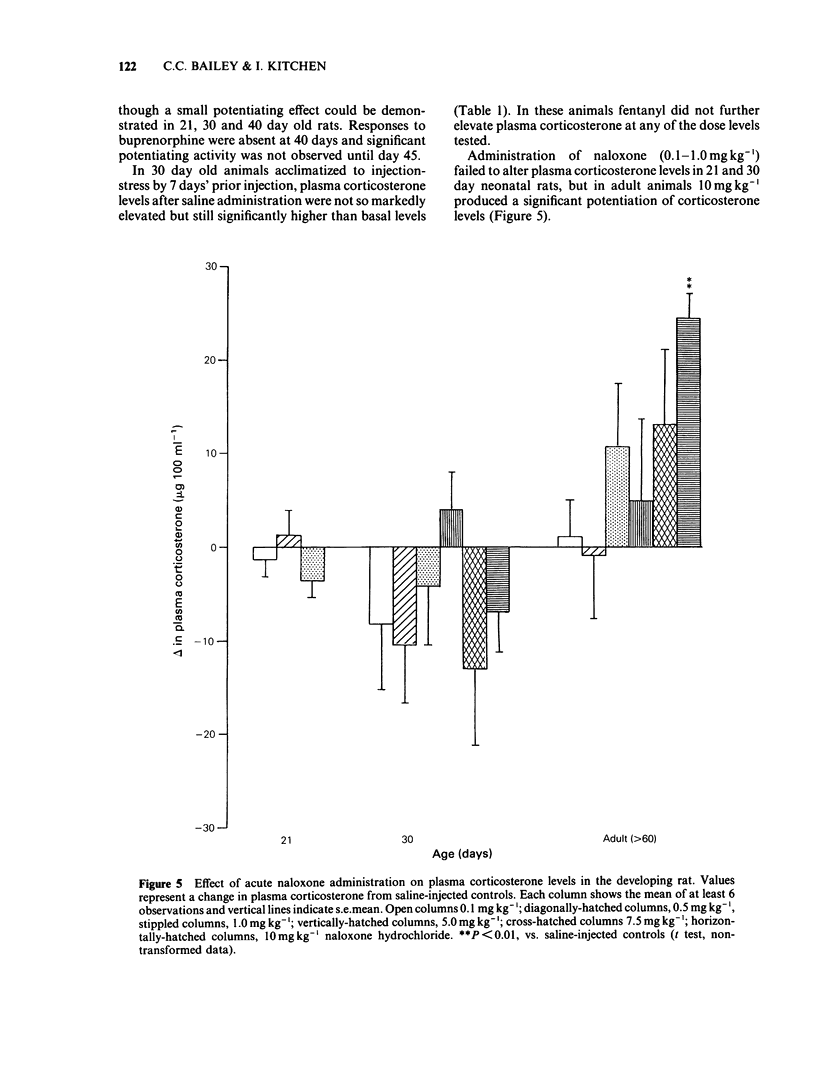
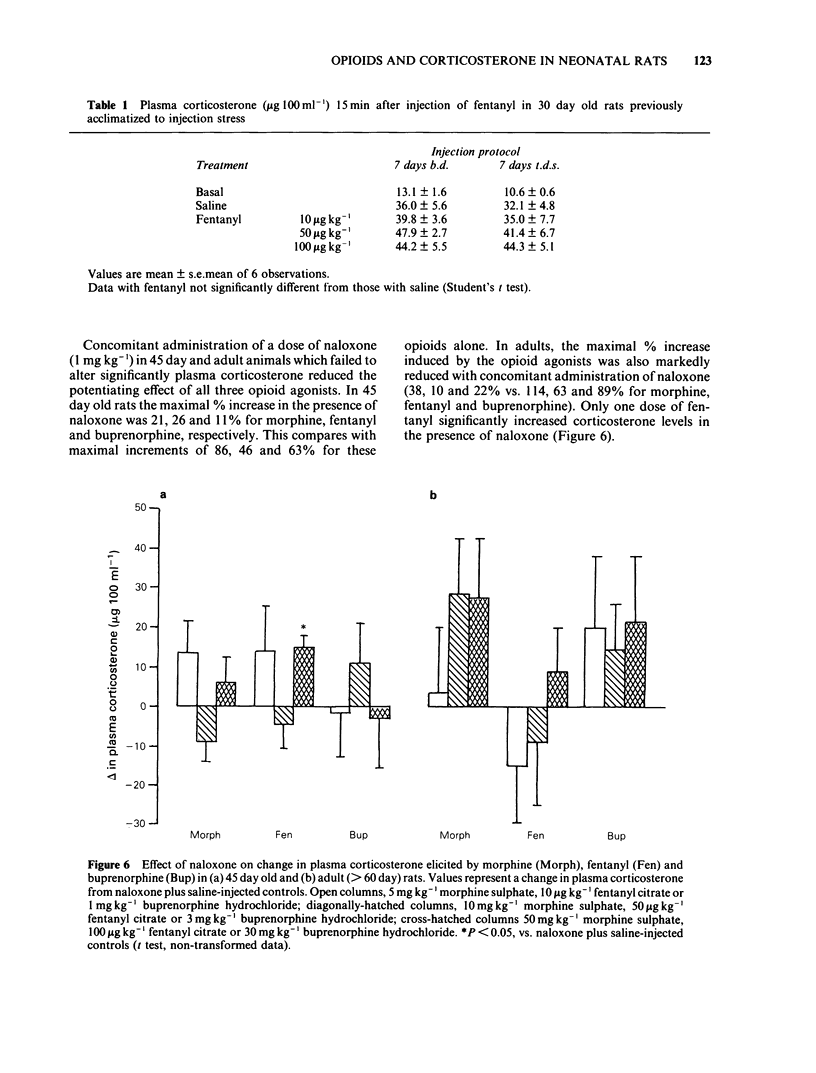
Abstract
The neonate has an unusual capacity for survival and the possibility exists that mechanisms for controlling stress responses may differ in the developing animal. In adults both endogenous and exogenous opioids can modulate the corticosterone responses to stress. We have studied this effect in neonatal rats and found that opioid modulation is absent in early postnatal development. Neonatal rats of either sex were injected with morphine (5-50 mg kg-1), fentanyl (10-100 micrograms kg-1), buprenorphine (0.1-30 mg kg-1) or naloxone (0.1-10 mg kg-1) and plasma corticosterone measured fluorimetrically 15 or 20 min later. In addition naloxone reversibility studies (1 mg kg-1, co-administered) were carried out for the opioid agonists. In adult rats, elevations in plasma corticosterone caused by injection stress were potentiated by morphine, fentanyl and buprenorphine. In neonates, though injection stress-induced rises in plasma corticosterone were absent at 10 days, elevations were observed at 21 days and later. However, significant potentiation of this corticosterone response by fentanyl was absent at 21 days and at later ages (30 and 40 days) for morphine and buprenorphine. The potentiating effect of all three agonists did not become fully effective until day 45. In addition, in animals acclimatized to injection stress by 7 day injection pretreatment, fentanyl did not significantly alter corticosterone levels in 30 day old neonates. High doses of naloxone (10 mg kg-1) significantly increased the corticosterone response to injection stress in adult rats but this effect was absent in 30 day old animals.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Kendall J. W. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967 May;80(5):926–930. doi: 10.1210/endo-80-5-926. [DOI] [PubMed] [Google Scholar]
- Bailey C., Kitchen I. Ontogenesis of proenkephalin products in rat striatum and the inhibitory effects of low-level lead exposure. Brain Res. 1985 Sep;354(1):75–79. doi: 10.1016/0165-3806(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Cowan A., Lewis J. W., Macfarlane I. R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977 Aug;60(4):537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Enna S. J. Neurochemical aspects of the ontogenesis of GABAnergic neurons in the rat brain. Brain Res. 1976 Jul 23;111(1):119–133. doi: 10.1016/0006-8993(76)91053-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. M. Effects of naloxone on plasma corticosterone in the opiate-naive rat. Life Sci. 1980 Mar 24;26(12):935–943. doi: 10.1016/0024-3205(80)90114-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. M. Plasma corticosterone changes in response to central or peripheral administration of kappa and sigma opiate agonists. J Pharmacol Exp Ther. 1985 Jun;233(3):863–869. [PubMed] [Google Scholar]
- Gibson A., Ginsburg M., Hall M., Hart S. L. The effects of opiate receptor agonists and antagonists on the stress-induced secretion of corticosterone in mice. Br J Pharmacol. 1979 Jan;65(1):139–146. doi: 10.1111/j.1476-5381.1979.tb17342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Ginsburg M., Hart S. L., Kitchen I. Effects of adrenalectomy and hypophysectomy on enkephalin content of the rat hypothalamus. Br J Pharmacol. 1980 Dec;70(4):561–569. doi: 10.1111/j.1476-5381.1980.tb09775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltmeyer G. C., Denenberg V. H., Thatcher J., Zarrow M. X. Response of the adrenal cortex of the neonatal rat after subjection to stress. Nature. 1966 Dec 17;212(5068):1371–1373. doi: 10.1038/2121371a0. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Stewart B. R. Effect of mu and kappa opioid receptor agonists on rat plasma corticosterone levels. Eur J Pharmacol. 1985 Oct 8;116(1-2):75–79. doi: 10.1016/0014-2999(85)90186-4. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978 Nov;235(5):E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Jezová D., Vigas M., Jurcovicová J. ACTH and corticosterone response to naloxone and morphine in normal, hypophysectomized and dexamethasone-treated rats. Life Sci. 1982 Jul 26;31(4):307–314. doi: 10.1016/0024-3205(82)90407-6. [DOI] [PubMed] [Google Scholar]
- Kakihana R., Blum S., Kessler S. Developmental study of pituitary-adrenocortical response in mice: plasma and brain corticosterone determination after histamine stress. J Endocrinol. 1974 Feb;60(2):353–358. doi: 10.1677/joe.0.0600353. [DOI] [PubMed] [Google Scholar]
- Kitchen I., McDowell J., Winder C., Wilson J. M. Low-level lead exposure alters morphine antinociception in neonatal rats. Toxicol Lett. 1984 Aug;22(2):119–123. doi: 10.1016/0378-4274(84)90054-7. [DOI] [PubMed] [Google Scholar]
- Kitchen I., Rowan K. M. Differences in the effects of mu- and delta-opioid receptor antagonists upon plasma corticosterone levels in stressed mice. Eur J Pharmacol. 1984 May 18;101(1-2):153–156. doi: 10.1016/0014-2999(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Kokka N., Garcia J. F., Elliott H. W. Effects of acute and chronic adminstration of narcotic analgesics on growth hormone and corticotrophin (ACTH) secretion in rats. Prog Brain Res. 1973;39:347–360. doi: 10.1016/s0079-6123(08)64091-1. [DOI] [PubMed] [Google Scholar]
- McDowell J., Kitchen I. Ontogenesis of delta-opioid receptors in rat brain using [3H][D-Pen2,D-Pen5]enkephalin as a binding ligand. Eur J Pharmacol. 1986 Sep 9;128(3):287–289. doi: 10.1016/0014-2999(86)90780-6. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Naitoh F., Segawa T. Regional changes in monoamine content and uptake of the rat brain during postnatal development. Brain Res. 1976 Jan 16;101(2):305–315. doi: 10.1016/0006-8993(76)90271-7. [DOI] [PubMed] [Google Scholar]
- Pechnick R., George R., Poland R. E. Identification of multiple opiate receptors through neuroendocrine responses. I. Effects of agonists. J Pharmacol Exp Ther. 1985 Jan;232(1):163–169. [PubMed] [Google Scholar]
- Pechnick R., George R., Poland R. E. Identification of multiple opiate receptors through neuroendocrine responses. II. Antagonism of mu, kappa and sigma agonists by naloxone and WIN 44,441-3. J Pharmacol Exp Ther. 1985 Jan;232(1):170–177. [PubMed] [Google Scholar]
- Rossier J., French E. D., Rivier C., Ling N., Guillemin R., Bloom F. E. Foot-shock induced stress increases beta-endorphin levels in blood but not brain. Nature. 1977 Dec 15;270(5638):618–620. doi: 10.1038/270618a0. [DOI] [PubMed] [Google Scholar]
- Rossier J., Guillemin R., Bloom F. Foot shock induced stress decreases leu5-enkephalin immunoreactivity in rat hypothalamus. Eur J Pharmacol. 1978 Apr 15;48(4):465–466. doi: 10.1016/0014-2999(78)90178-4. [DOI] [PubMed] [Google Scholar]
- Siegel R. A., Chowers I., Conforti N., Feldman S., Weidenfeld J. Effects of naloxone on basal and stress-induced ACTH and corticosterone secretion in the male rat--site and mechanism of action. Brain Res. 1982 Oct 7;249(1):103–109. doi: 10.1016/0006-8993(82)90174-3. [DOI] [PubMed] [Google Scholar]
- Spain J. W., Roth B. L., Coscia C. J. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa). J Neurosci. 1985 Mar;5(3):584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp W. N., Mittler J. C., Natelson B. H. Effects of naloxone on corticosterone response to stress. Pharmacol Biochem Behav. 1981 May;14(5):749–751. doi: 10.1016/0091-3057(81)90143-x. [DOI] [PubMed] [Google Scholar]
- Walker C. D., Perrin M., Vale W., Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986 Apr;118(4):1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]


