Abstract
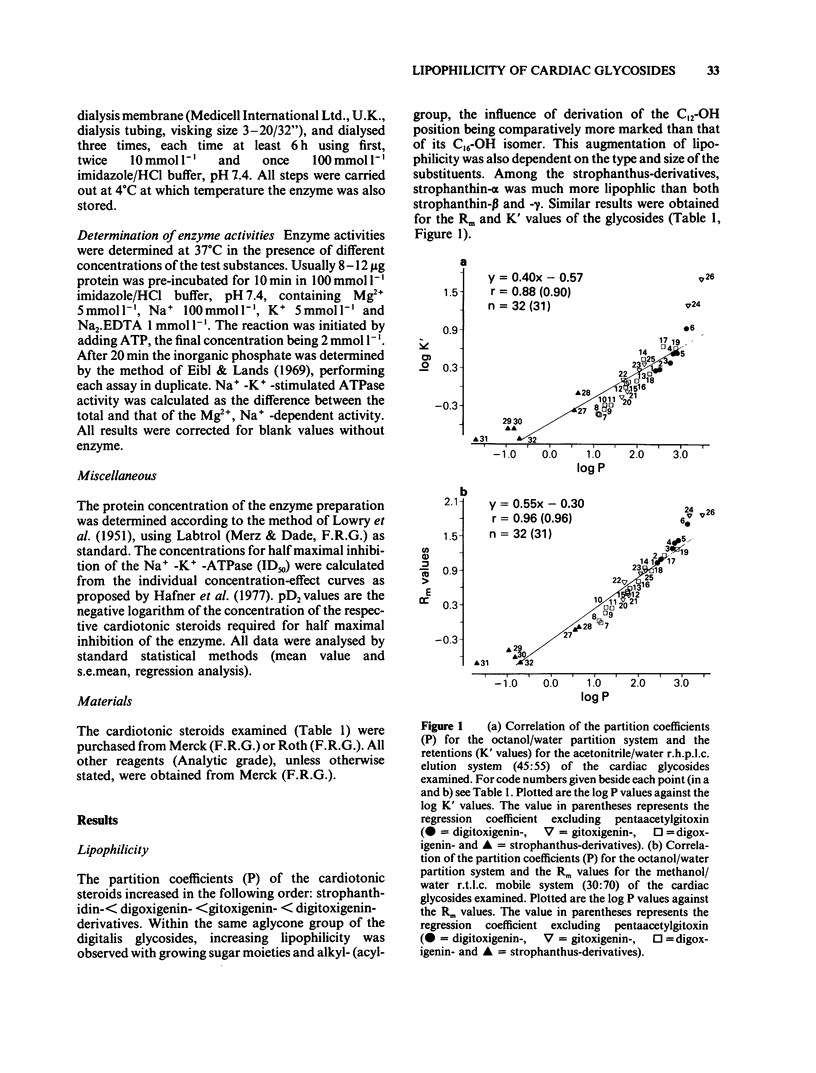
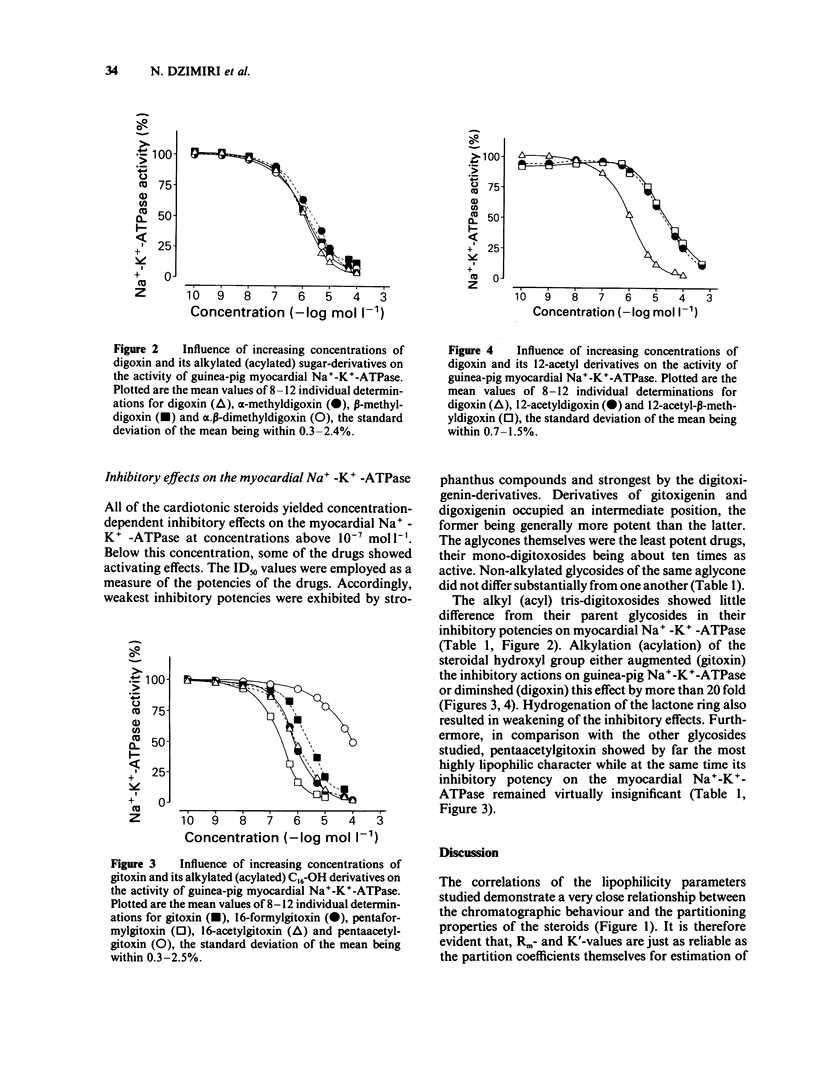
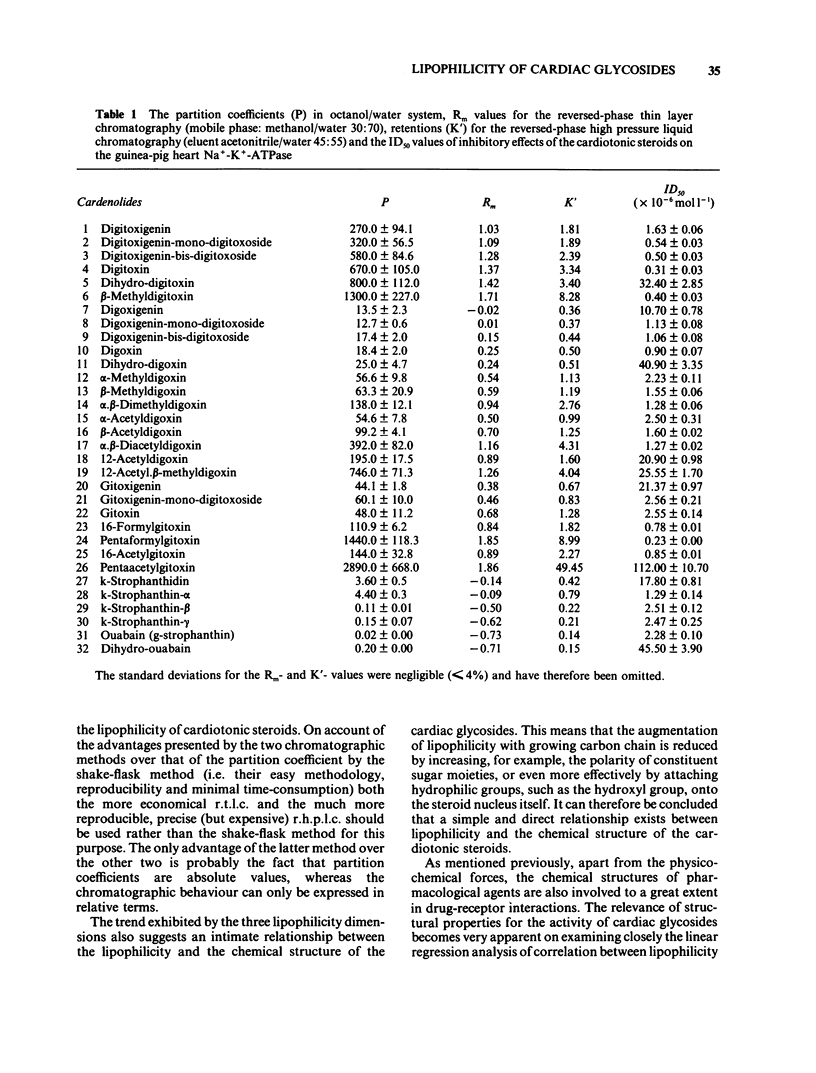
Lipophilicity and inhibitory actions on guinea-pig heart Na+-K+-ATPase of twenty-six digitalis and six strophanthus glycosides comprising the aglycones, mono-, bis-, tris-sugar, alkylated (acylated) tris-sugar, acyl steroid derivatives and three cardanolides were investigated. Their octanol/water partition coefficients (P), reversed phase thin layer (r.t.l.c.) and reversed phase high performance liquid chromatography (r.h.p.l.c.) were determined and the viability of these methods as a measure of the lipophilicity of the cardiotonic steroids evaluated. The influence of lipophilicity and so also structural changes on the inhibitory effects of the cardiac glycosides on myocardial Na+-K+-ATPase was then examined. It is concluded that (a) r.t.l.c. and r.h.p.l.c. are just as effective as the conventional shake-flask method for estimation of the lipophilicity of cardiac glycosides and (b) the inhibitory potencies of cardiotonic steroids on the myocardial Na+-K+-ATPase increase with growing lipophilicity. The relationship between these two parameters is, however, governed by the influence of substitution or derivation of structural components on their inhibitory potencies on the myocardial Na+-K+-ATPase.
Full text
PDF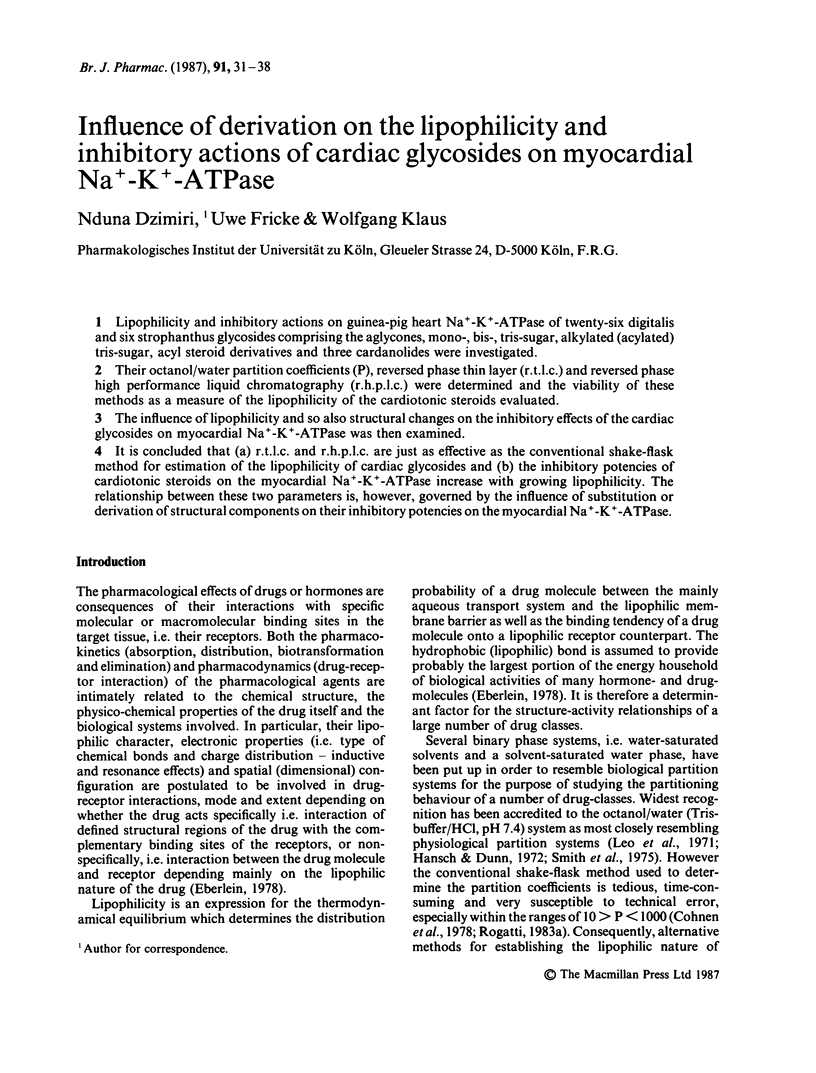



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. K., Skelton R. E., Riley T. N., Bagley J. R. Estimation of high pressure liquid chromatographic retention indices of narcotic analgetics and related drugs. J Chromatogr Sci. 1980 Apr;18(4):153–158. doi: 10.1093/chromsci/18.4.153. [DOI] [PubMed] [Google Scholar]
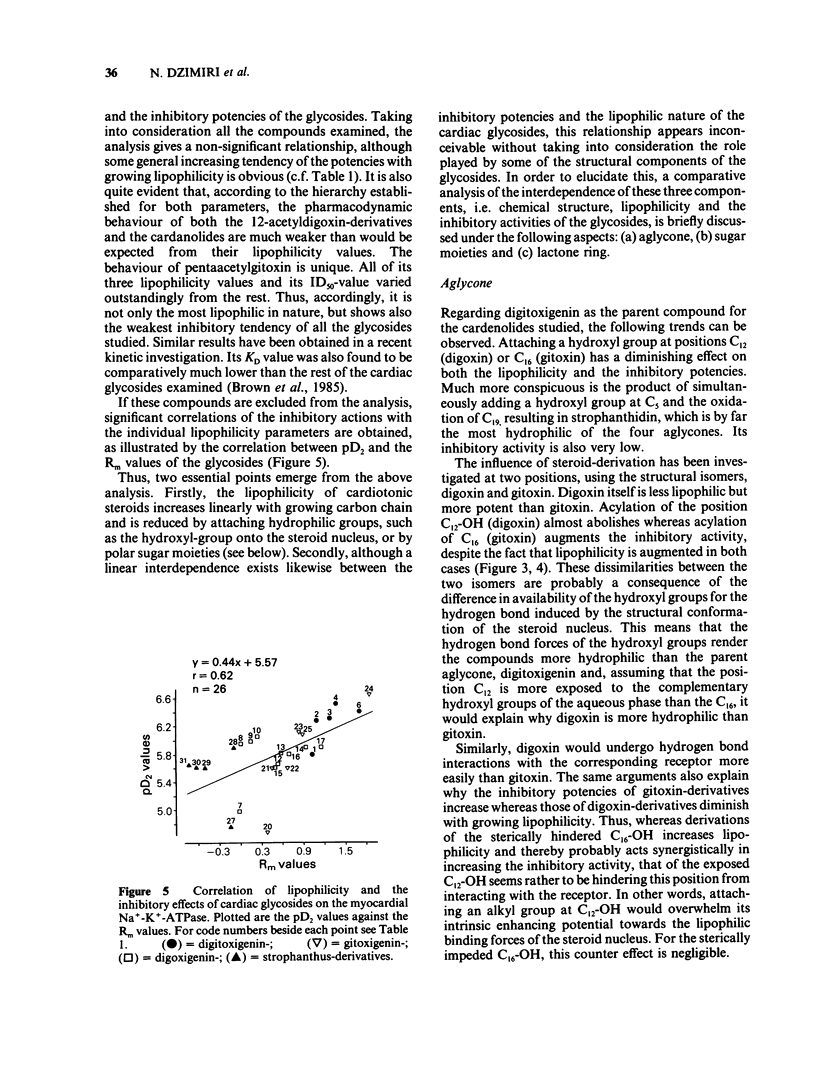
- Brown L., Hug E., Wagner G., Erdmann E. Comparison of ouabain receptors in sheep myocardium and Purkinje fibres. Biochem Pharmacol. 1985 Oct 15;34(20):3701–3710. doi: 10.1016/0006-2952(85)90234-5. [DOI] [PubMed] [Google Scholar]
- CHEN K. K., HENDERSON F. G. Pharmacology of sixty-four cardiac glycosides and aglycones. J Pharmacol Exp Ther. 1954 Jul;111(3):365–383. [PubMed] [Google Scholar]
- Cohnen E., Flasch H., Heinz N., Hempelmann F. W. Verteilungskoeffizienten und Rm-Werte von Cardenoliden. Arzneimittelforschung. 1978;28(12):2179–2182. [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Fricke U., Klaus W. A simple preparation technique for a microsomal Maplus-K-plus-activated ATPase from cardiac tissues of different species. Prep Biochem. 1974;4(1):13–29. doi: 10.1080/00327487408068183. [DOI] [PubMed] [Google Scholar]
- Hafner D., Heinen E., Noack E. Mathematical analysis of concentration-response relationships. Method for the evaluation of the ED50 and the number of binding sites per receptor molecule using the logit transformation. Arzneimittelforschung. 1977;27(10):1871–1873. [PubMed] [Google Scholar]
- Hansch C., Dunn W. J., 3rd Linear relationships between lipophilic character and biological activity of drugs. J Pharm Sci. 1972 Jan;61(1):1–19. doi: 10.1002/jps.2600610102. [DOI] [PubMed] [Google Scholar]
- Hempelmann F. W., Heinz N., Flasch H. Lipophilie und enterale Resorption bei Cardenoliden. Arzneimittelforschung. 1978;28(12):2182–2185. [PubMed] [Google Scholar]
- Henry D., Block J. H., Anderson J. L., Carlson G. R. Use of high-pressure liquid chromatography for quantitative structure-activity relationship studies of sulfonamides and barbiturates. J Med Chem. 1976 May;19(5):619–626. doi: 10.1021/jm00227a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCall J. M. Liquid-lipquid partition coefficients by high-pressure liquid chromatography. J Med Chem. 1975 Jun;18(6):549–552. doi: 10.1021/jm00240a003. [DOI] [PubMed] [Google Scholar]
- Mirrlees M. S., Moulton S. J., Murphy C. T., Taylor P. J. Direct measurement of octanol-water partition coefficients by high-pressure liquid chromatography. J Med Chem. 1976 May;19(5):615–619. doi: 10.1021/jm00227a008. [DOI] [PubMed] [Google Scholar]
- Repke K. R. Biochemische Grundlagen der Entwicklung neuartiger Herzmittel des Digitalistyps. Pharmazie. 1972 Nov;27(11):693–701. [PubMed] [Google Scholar]
- Smith R. N., Hansch C., Ames M. M. Selection of a reference partitioning system for drug design work. J Pharm Sci. 1975 Apr;64(4):599–606. doi: 10.1002/jps.2600640405. [DOI] [PubMed] [Google Scholar]
- Unger S. H., Cook J. R., Hollenberg J. S. Simple procedure for determining octanol--aqueous partition, distribution, and ionization coefficients by reversed-phase high-pressure liquid chromatography. J Pharm Sci. 1978 Oct;67(10):1364–1367. doi: 10.1002/jps.2600671008. [DOI] [PubMed] [Google Scholar]
- Yoda A., Yoda S. Association and dissociation rate constants of the complexes between various cardiac aglycones and sodium- and potassium-dependent adenosine triphosphatase formed in the presence of magnesium and phosphate. Mol Pharmacol. 1977 Mar;13(2):352–361. [PubMed] [Google Scholar]


