Abstract
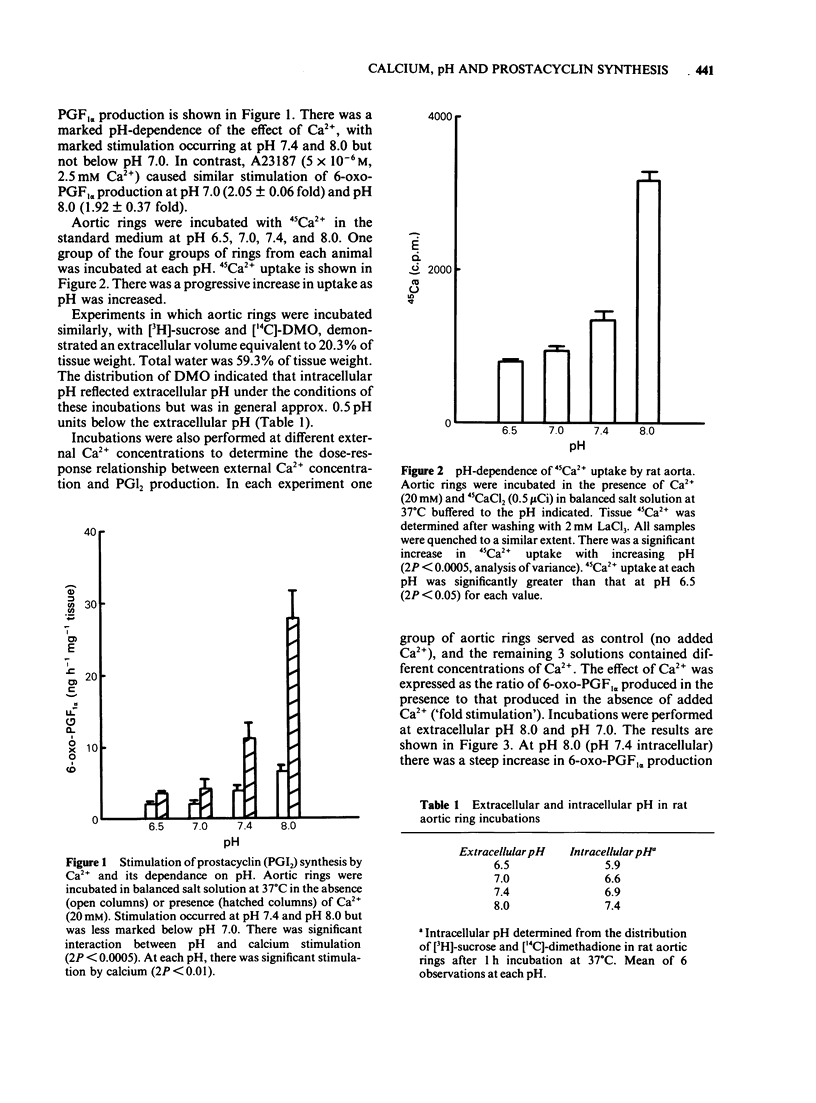
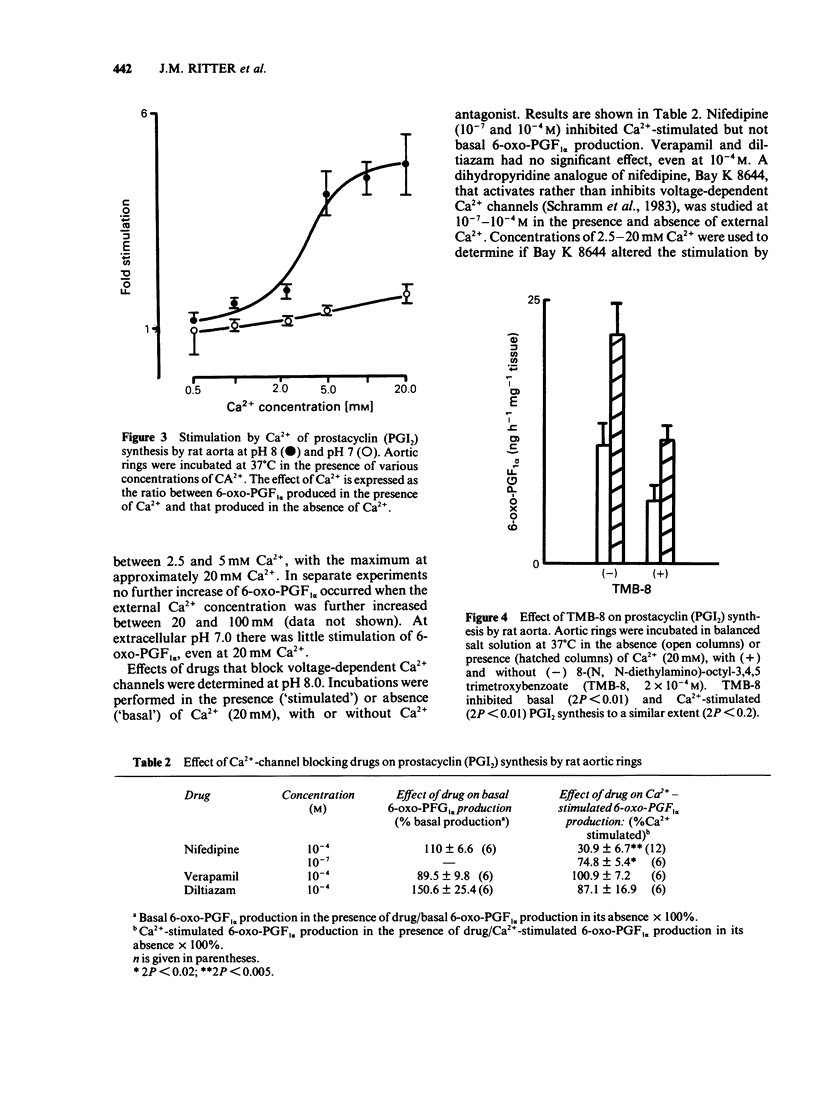
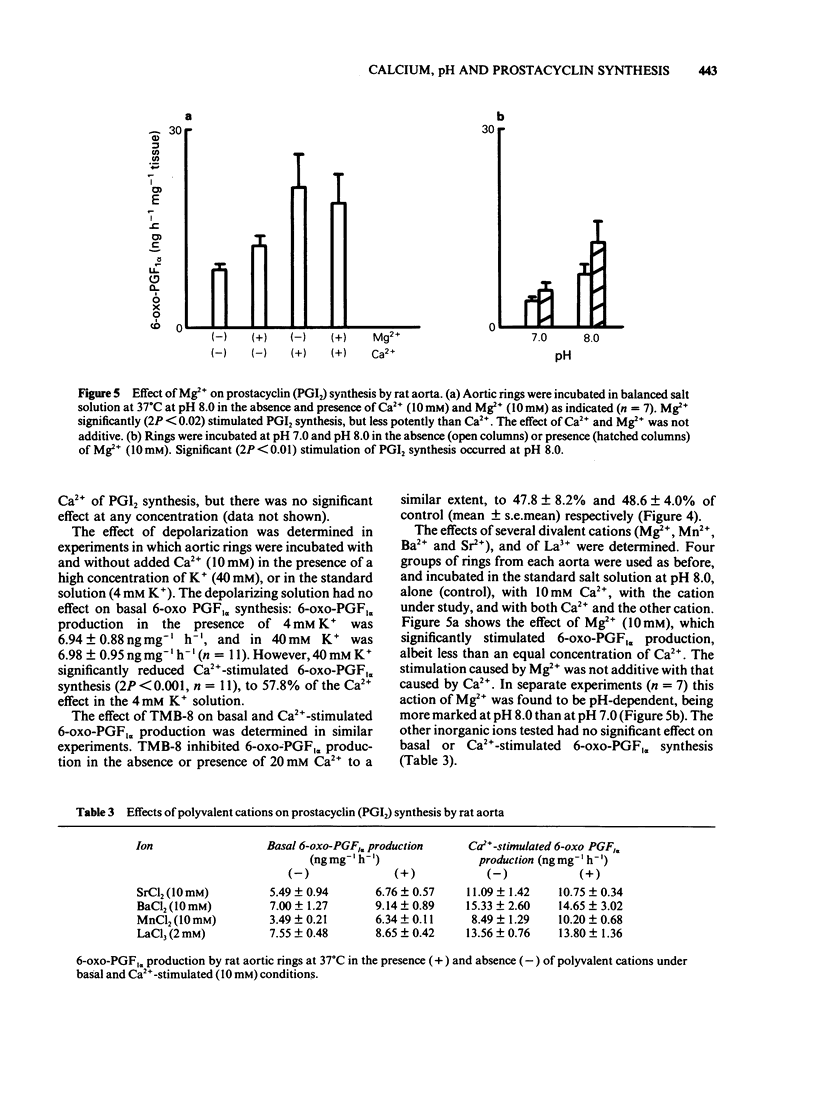
Fresh rat aortic rings were incubated in HEPES-buffered salt solutions. Extracellular Ca2+ stimulated the production of prostacyclin (PGI2), as determined by radioimmunoassay of its stable hydrolysis product 6-oxo-prostaglandin F1 alpha. This action of Ca2+ was modified by H+ over the pH range 8.0-6.5. Stimulation by calcium ionophore A23187 was not pH-dependent. In parallel incubations of aortic rings with 45Ca2+, followed by washing in the presence of La3+, tissue uptake of 45Ca2+ increased progressively as extracellular pH was increased from 6.5-8.0. Over this range intracellular pH, estimated by the distribution of [14C]-dimethadione, varied from 5.9-7.4. Stimulation by Ca2+ of PGI2 synthesis was concentration-dependent over the range 0.7-20 mM. The maximum effect was an increase of approx. 4 fold. Nifedipine, but not verapamil or diltiazem, inhibited Ca2+-stimulated PGI2 synthesis. A dihydropyridine compound that activates voltage-dependent Ca2+ channels, Bay K 8644, did not increase PGI2 synthesis. 8-(N, N-diethylamino)-octyl-3,4,5 timethoxybenzoate (TMB-8), an antagonist of intracellular Ca2+ mobilisation, inhibited basal and Ca2+-stimulated PGI2 synthesis to a similar extent. A solution containing 40 mM K+ reduced Ca2+-stimulated PGI2 production. Mg2+ stimulated PGI2 synthesis in a pH-dependent manner but was less potent than Ca2+. Other divalent cations (Mn2+, Ba2+ and Sr2+), and La3+ had little or no effect on basal or Ca2+-stimulated PGI2 synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. The use of lanthanum to estimate the numbers of extracellular cation-exchanging sites in the guinea-pig's taenia coli, and its effects on transmembrane monovalent ion movements. J Physiol. 1977 Apr;266(2):255–273. doi: 10.1113/jphysiol.1977.sp011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Frank G. B. The current view of the source of trigger calcium in excitation-contraction coupling in vertebrate skeletal muscle. Biochem Pharmacol. 1980 Sep 15;29(18):2399–2406. doi: 10.1016/0006-2952(80)90341-x. [DOI] [PubMed] [Google Scholar]
- Hassid A., Oudinet J. P. Relationship between cellular calcium and prostaglandin synthesis in cultured vascular smooth muscle cells. Prostaglandins. 1986 Sep;32(3):457–478. doi: 10.1016/0090-6980(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Hensby C. N., Jogee M., Elder M. G., Myatt L. A comparison of the quantitative analysis of 6-oxo-PGF1 alpha in biological fluids by gas chromatography mass spectrometry and radioimmunoassay. Biomed Mass Spectrom. 1981 Mar;8(3):111–117. doi: 10.1002/bms.1200080306. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Adrenergic modulation of vascular prostacyclin (PGI2) secretion. Eur J Pharmacol. 1985 Aug 7;114(1):33–40. doi: 10.1016/0014-2999(85)90517-5. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. The effect of nifedipine, nimodipine and nisoldipine on agonist- and trauma-stimulated vascular prostacyclin synthesis in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):70–73. doi: 10.1007/BF00633200. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Selective inhibition of the transmembrane Ca conductivity of mammalian myocardial fibres by Ni, Co and Mn ions. Pflugers Arch. 1973 Jan 22;338(2):115–123. doi: 10.1007/BF00592747. [DOI] [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca++ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in skeletal muscles. Pharmacology. 1974;12(1):20–31. doi: 10.1159/000136517. [DOI] [PubMed] [Google Scholar]
- Mehta J., Mehta P., Ostrowski N. Calcium blocker diltiazem inhibits platelet activation and stimulates vascular prostacyclin synthesis. Am J Med Sci. 1986 Jan;291(1):20–24. doi: 10.1097/00000441-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979 May 17;300(20):1142–1147. doi: 10.1056/NEJM197905173002006. [DOI] [PubMed] [Google Scholar]
- Nadler J. L., McKay M., Campese V., Vrbanac J., Horton R. Evidence that prostacyclin modulates the vascular actions of calcium in man. J Clin Invest. 1986 Apr;77(4):1278–1284. doi: 10.1172/JCI112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ritter J. M., Orchard M. A., Blair I. A., Lewis P. J. The time course and magnitude of prostacyclin (PGI2) production by rat aortic rings incubated in human plasma. Biochem Pharmacol. 1982 Mar 15;31(6):1163–1165. doi: 10.1016/0006-2952(82)90361-6. [DOI] [PubMed] [Google Scholar]
- Ritter J. M., Orchard M. A., Lewis P. J. Stimulation of vascular prostacyclin (PGI2) production by human serum. Biochem Pharmacol. 1982 Oct 1;31(19):3047–3050. doi: 10.1016/0006-2952(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Ritter J. M. Prostanoid synthesis by aortic rings in human blood: selective increase of prostacyclin mediated by a serum factor. Br J Pharmacol. 1984 Oct;83(2):409–418. doi: 10.1111/j.1476-5381.1984.tb16501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Properties and distribution of phosphatidylinositol-specific phospholipase C in human and horse platelets. Biochim Biophys Acta. 1983 Jul 12;752(2):329–338. doi: 10.1016/0005-2760(83)90131-5. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. Low pH selectively blocks calcium action potentials in amphibian neurons developing in culture. Brain Res. 1979 Feb 9;161(3):555–559. doi: 10.1016/0006-8993(79)90687-5. [DOI] [PubMed] [Google Scholar]
- Taylor G. W., Chappell C. G., Frazer C. E., Ritter J. M. Prostacyclin synthesis by vascular tissue. Effect of extracellular Ca2+. Biochem J. 1986 Oct 1;239(1):237–239. doi: 10.1042/bj2390237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Van de Velde V. J., Van den Bossche R. M., Bult H., Herman A. G. Modulation of prostacyclin biosynthesis by calcium entry blockers and extracellular calcium. Biochem Pharmacol. 1986 Jan 15;35(2):253–256. doi: 10.1016/0006-2952(86)90522-8. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N. Blockade of myocardial slow inward current at low pH. Am J Physiol. 1977 Sep;233(3):C99–103. doi: 10.1152/ajpcell.1977.233.3.C99. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton A. R., Willis C. E., Kent R. S., Young S. L. The role of calcium in the regulation of prostacyclin synthesis by porcine aortic endothelial cells. Lipids. 1984 Jan;19(1):17–24. doi: 10.1007/BF02534603. [DOI] [PubMed] [Google Scholar]


