Abstract
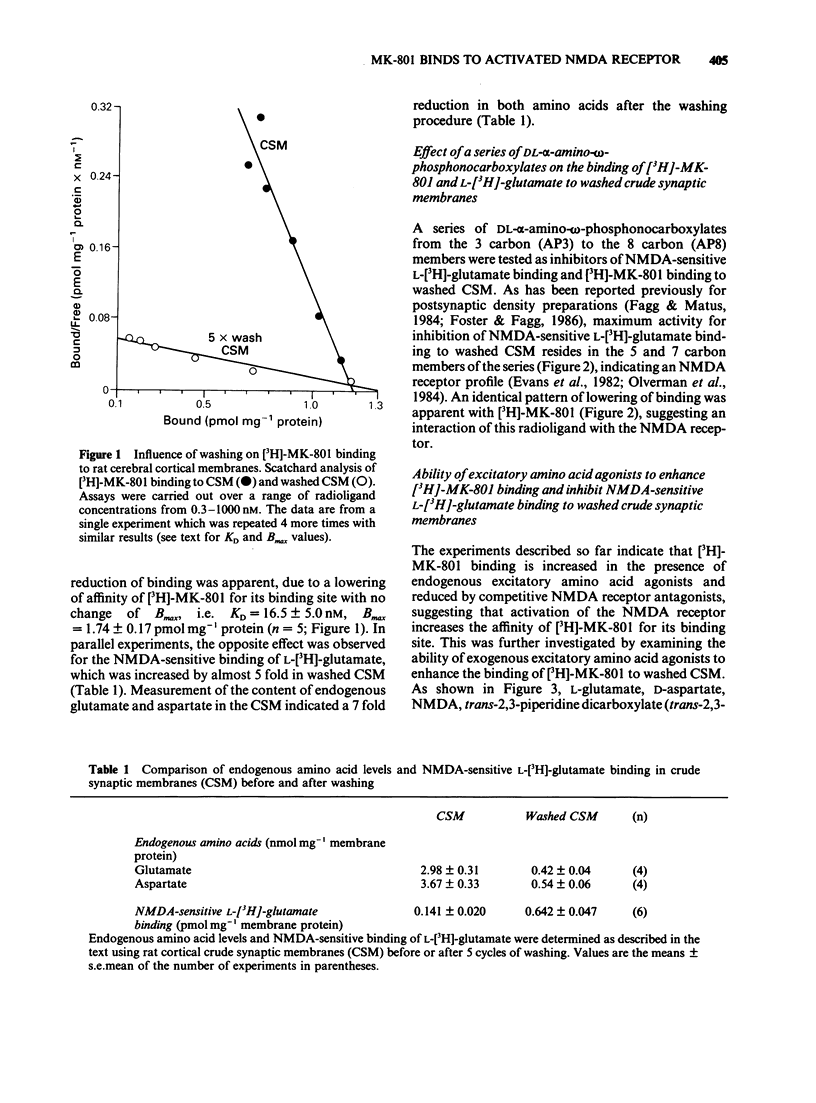
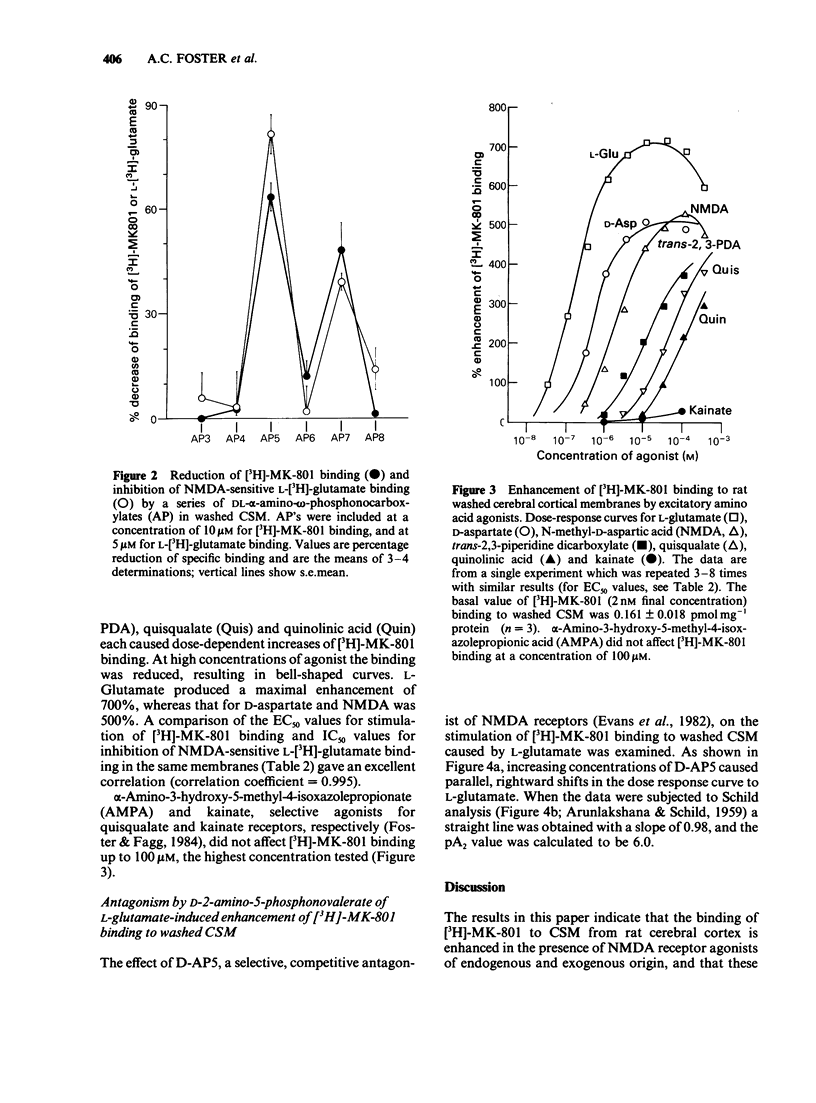
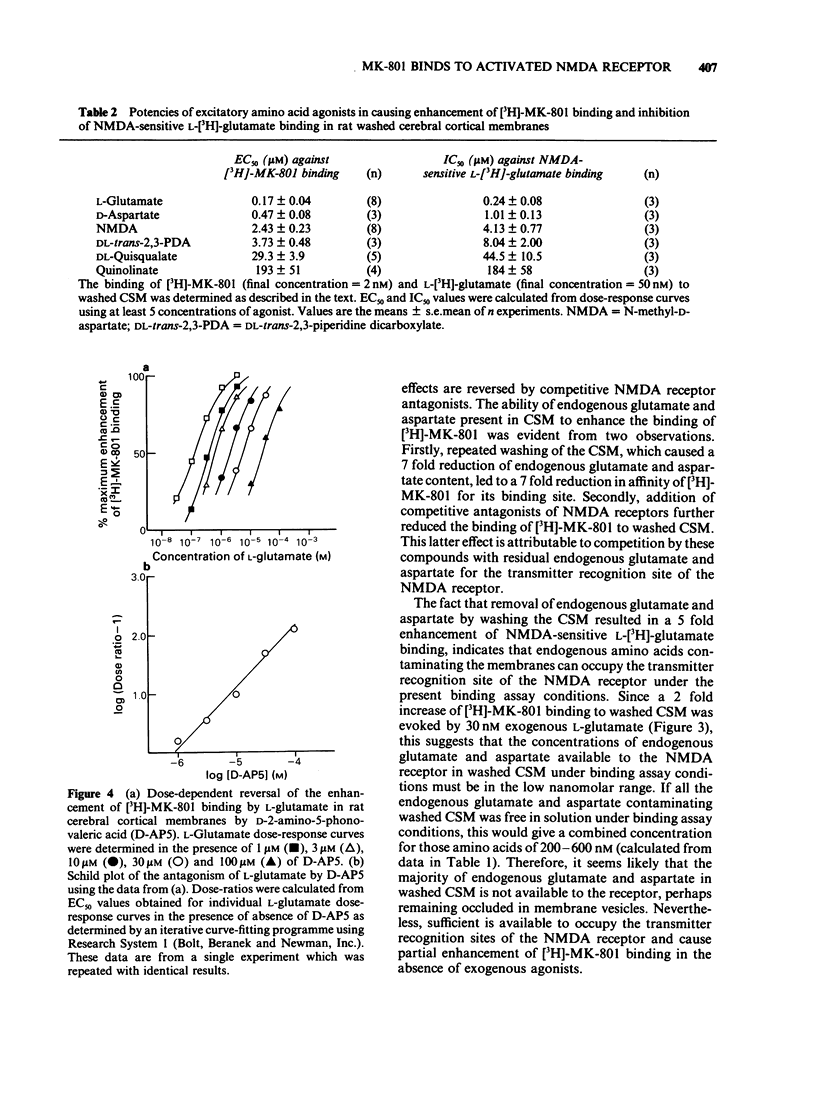
The influence of endogenous and exogenous acidic amino acids on the binding of [3H]-MK-801, a selective, non-competitive antagonist of N-methyl-D-aspartate (NMDA) receptors, has been investigated in rat cerebral cortex crude synaptic membranes (CSM). Removal of endogenous glutamate and aspartate from CSM by repeated washing reduced the affinity of [3H]-MK-801 for its binding site (with no change in the total number of binding sites) and increased NMDA-sensitive L-[3H]-glutamate binding. In washed CSM, competitive NMDA antagonists of the DL-alpha-amino-omega-phosphonocarboxylate series reduced [3H]-MK-801 binding and NMDA-sensitive L-[3H]-glutamate binding, the most active compounds being 2-amino-5-phosphonovalerate (AP5) and 2-amino-7-phosphono-heptanoate (AP7). Exogenous excitatory amino acid agonists enhanced the binding of [3H]-MK-801 to washed CSM by up to 700%. A selective involvement of NMDA receptors in these effects was indicated by the excellent correlation between EC50s for stimulation of [3H]-MK-801 binding and IC50s for inhibition of NMDA-sensitive L-[3H]-glutamate binding in the same membranes. The selective, competitive NMDA receptor antagonist D-AP5 blocked the L-glutamate-induced increase in [3H]-MK-801 binding in a competitive manner with a pA2 value of 6.0. These results seem to reflect a molecular interaction between two distinct components of the NMDA receptor complex: the transmitter recognition site and the site through which MK-801 exerts its antagonist effects, possibly the ion channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Aronstam R. S., Bakry N. M., Eldefrawi A. T., Albuquerque E. X. Activation, inactivation, and desensitization of acetylcholine receptor channel complex detected by binding of perhydrohistrionicotoxin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2309–2313. doi: 10.1073/pnas.77.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Aronstam R. S., Maleque M. A., Warnick J. E., Albuquerque E. X. [3H]Phencyclidine: a probe for the ionic channel of the nicotinic receptor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7458–7462. doi: 10.1073/pnas.77.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Comparison of L-[3H]glutamate, D-[3H]aspartate, DL-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol. 1987 Jan 20;133(3):291–300. doi: 10.1016/0014-2999(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Olson J. M., Penney J. B., Jr, Young A. B. Autoradiographic characterization of N-methyl-D-aspartate-, quisqualate- and kainate-sensitive glutamate binding sites. J Pharmacol Exp Ther. 1985 Apr;233(1):254–263. [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loo P., Braunwalder A., Lehmann J., Williams M. Radioligand binding to central phencyclidine recognition sites is dependent on excitatory amino acid receptor agonists. Eur J Pharmacol. 1986 Apr 29;123(3):467–468. doi: 10.1016/0014-2999(86)90726-0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Cotman C. W. L-[3H]Glutamate binds to kainate-, NMDA- and AMPA-sensitive binding sites: an autoradiographic analysis. Brain Res. 1985 Aug 12;340(2):378–383. doi: 10.1016/0006-8993(85)90936-9. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Iversen L. L. Modulation of [3H]diazepam binding in rat cortical membranes by GABAA agonists. J Neurochem. 1985 Apr;44(4):1162–1167. doi: 10.1111/j.1471-4159.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


