Abstract
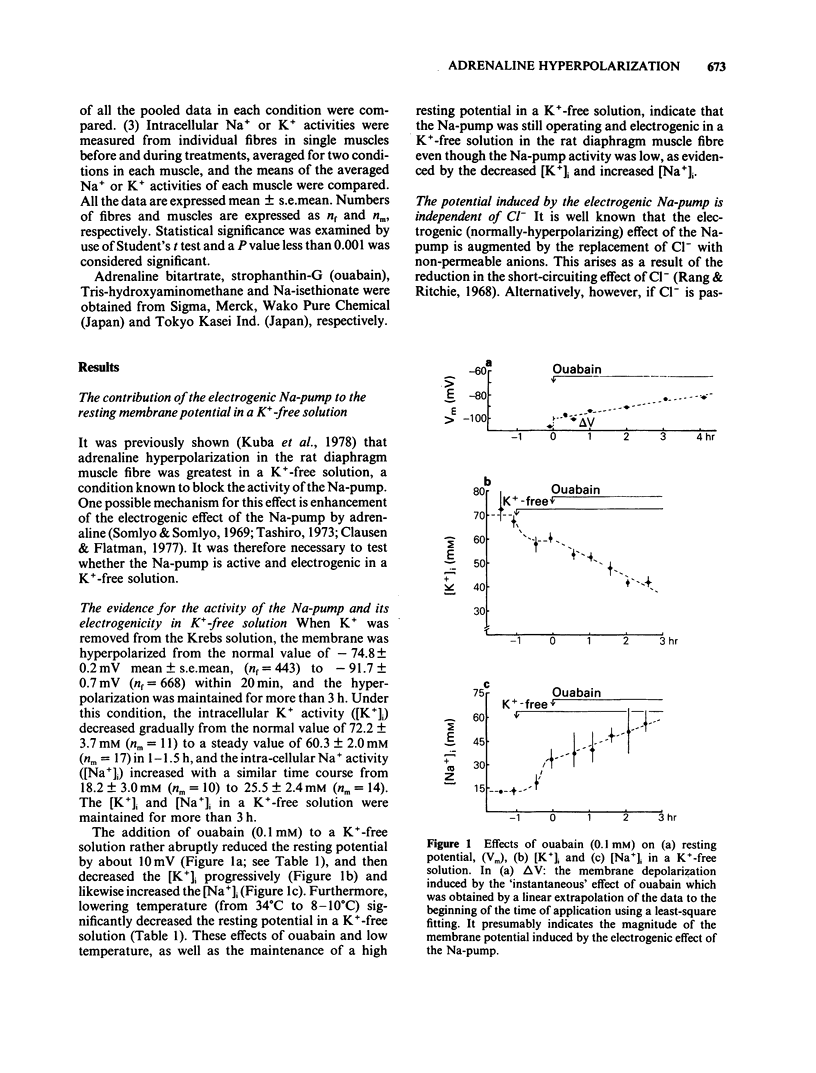
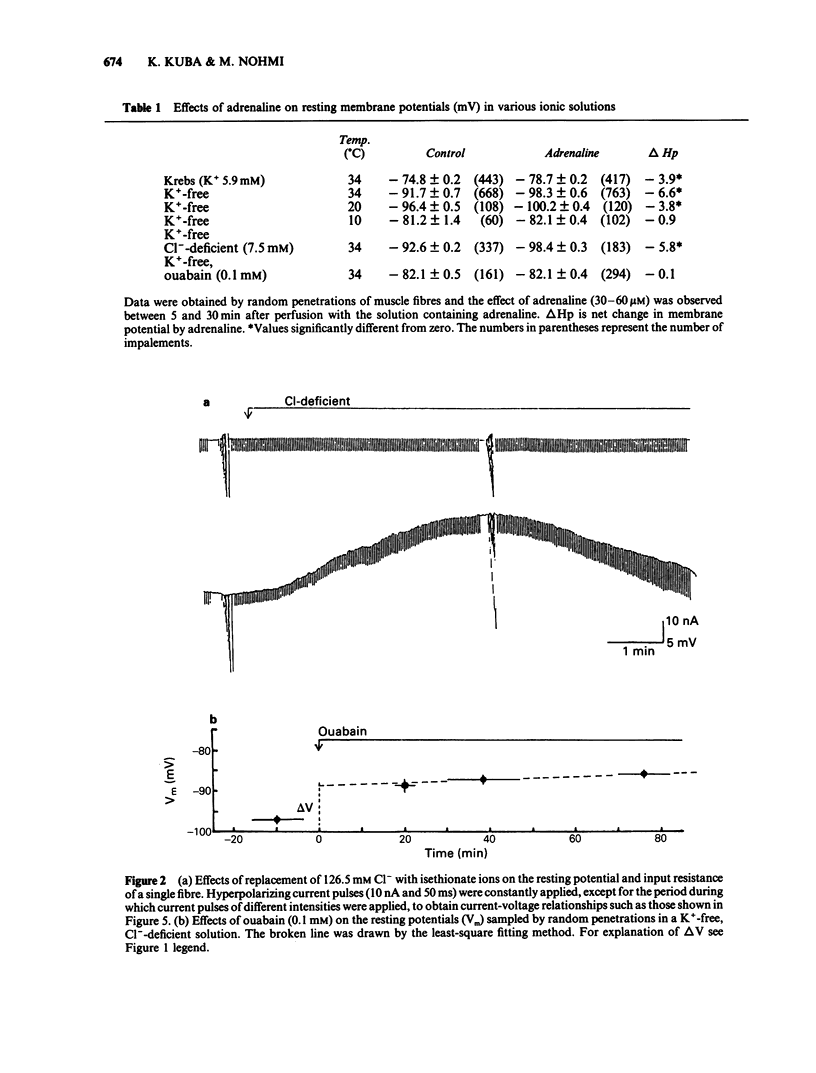
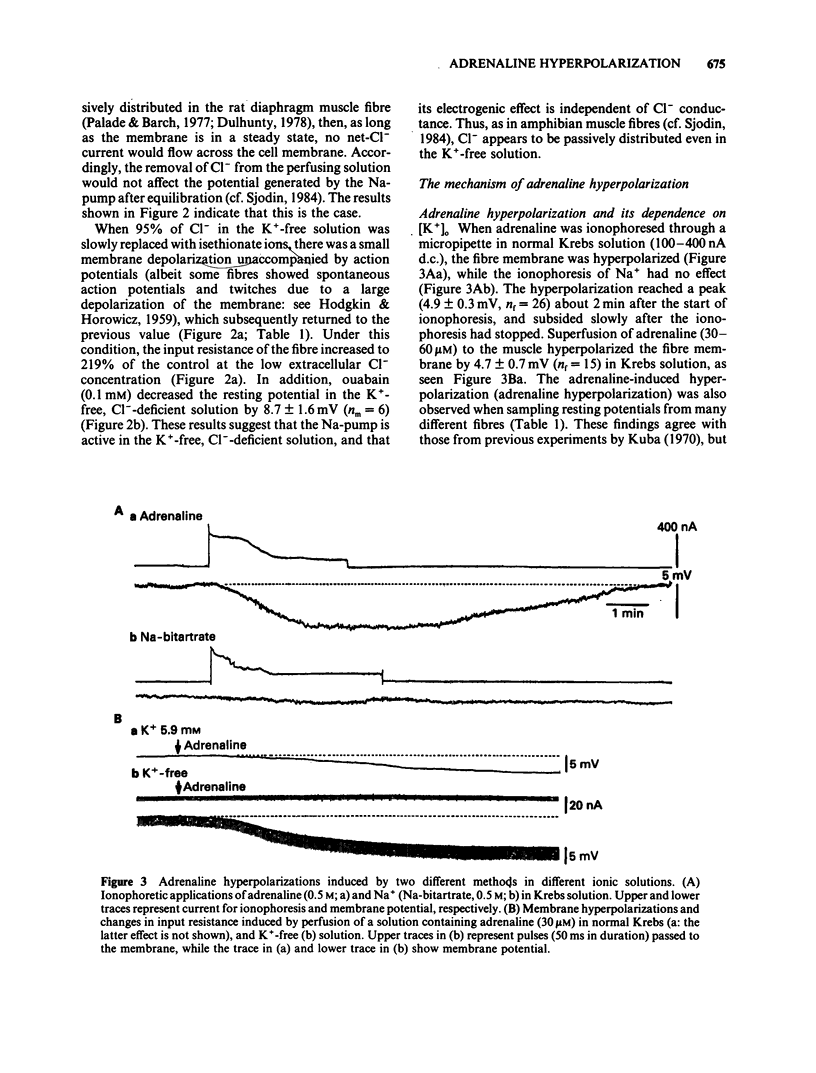
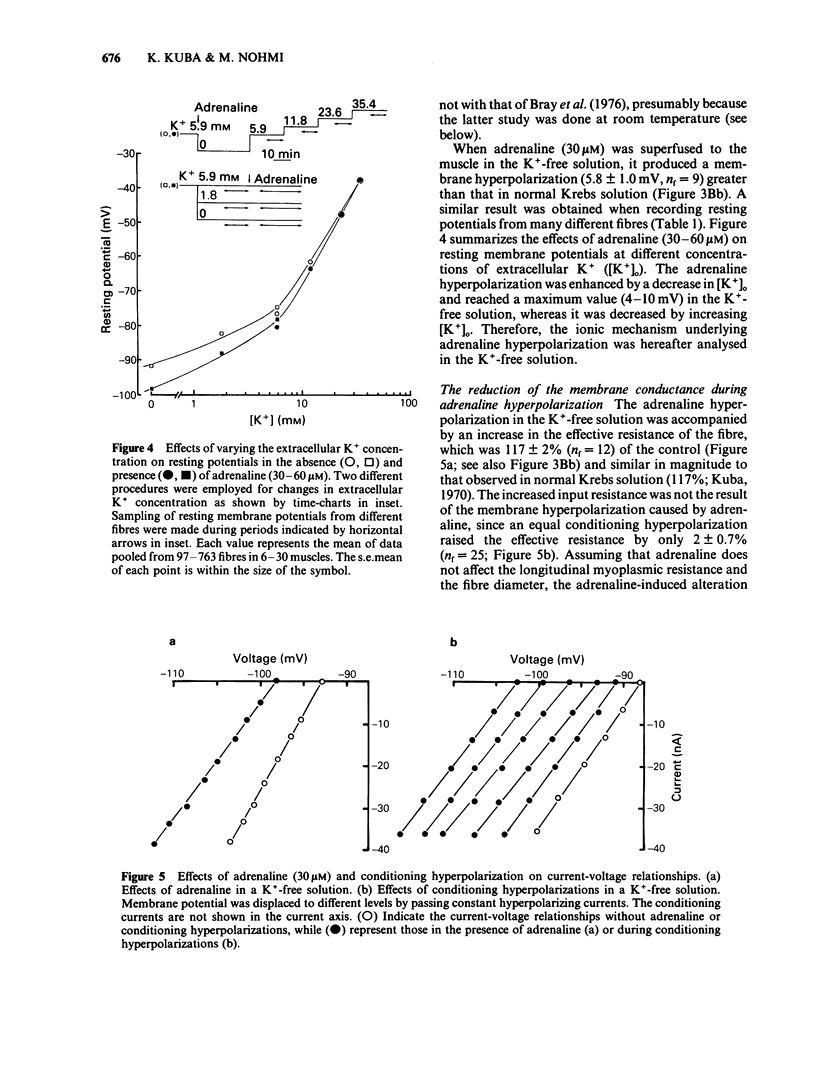
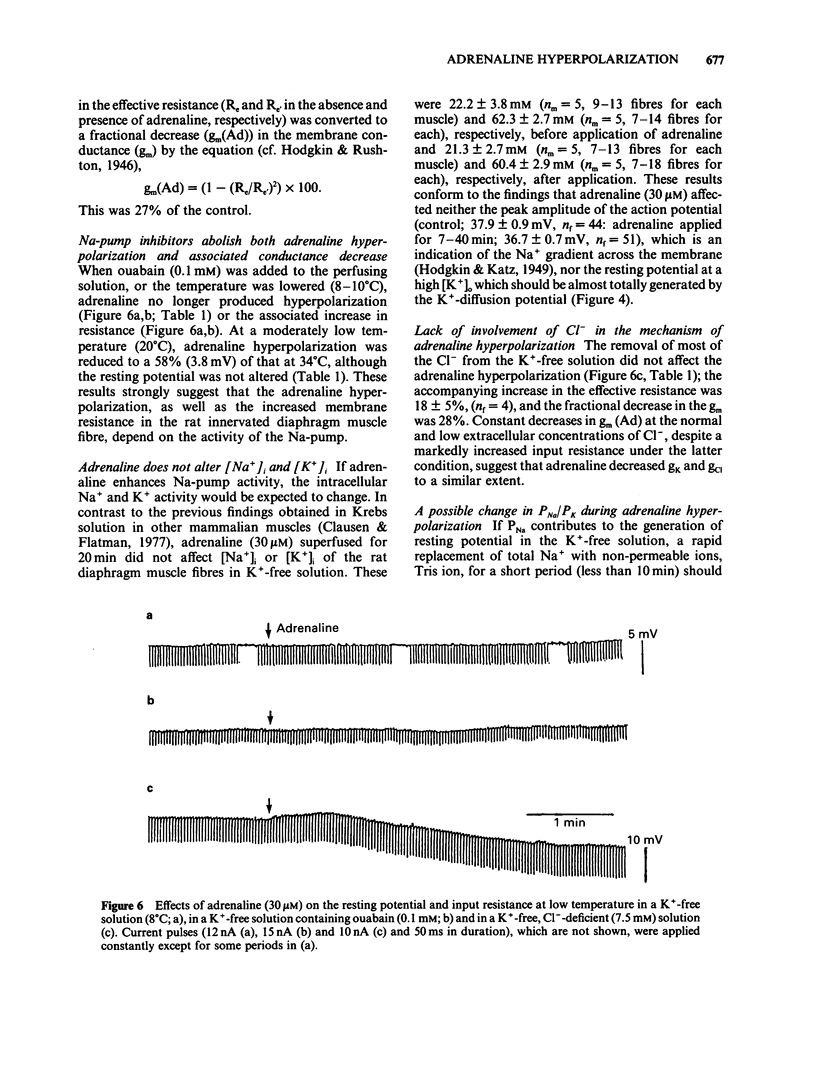
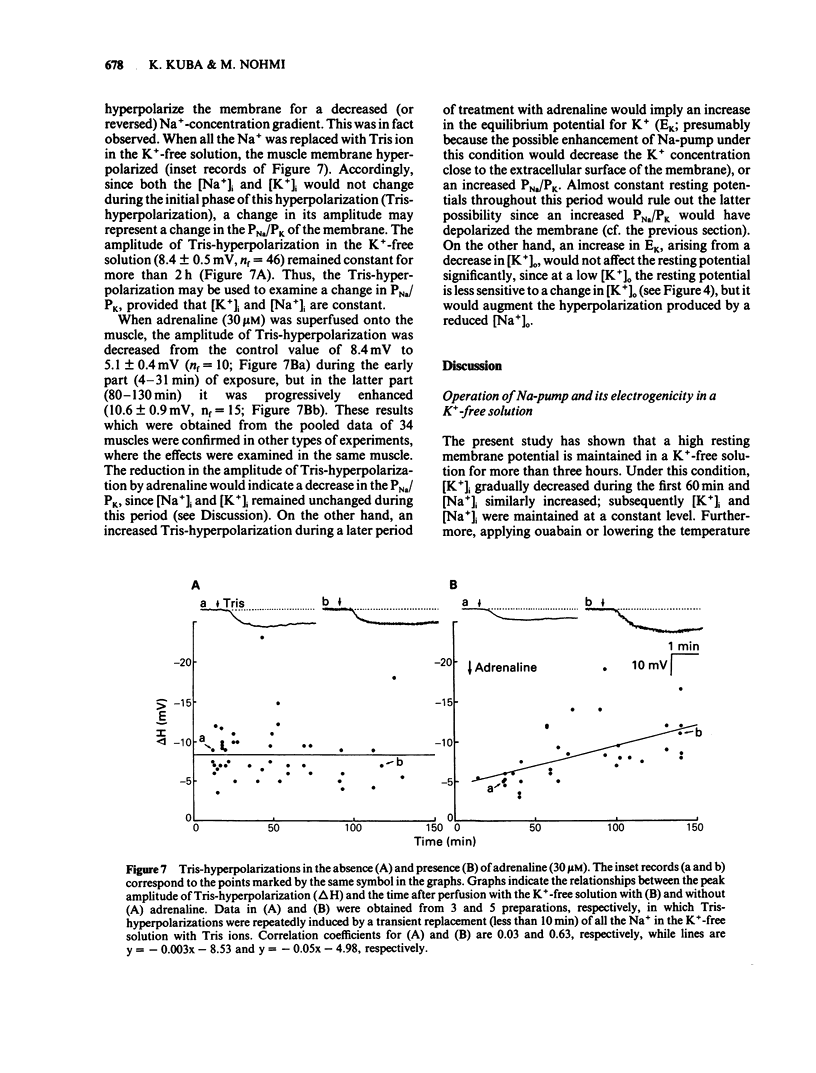
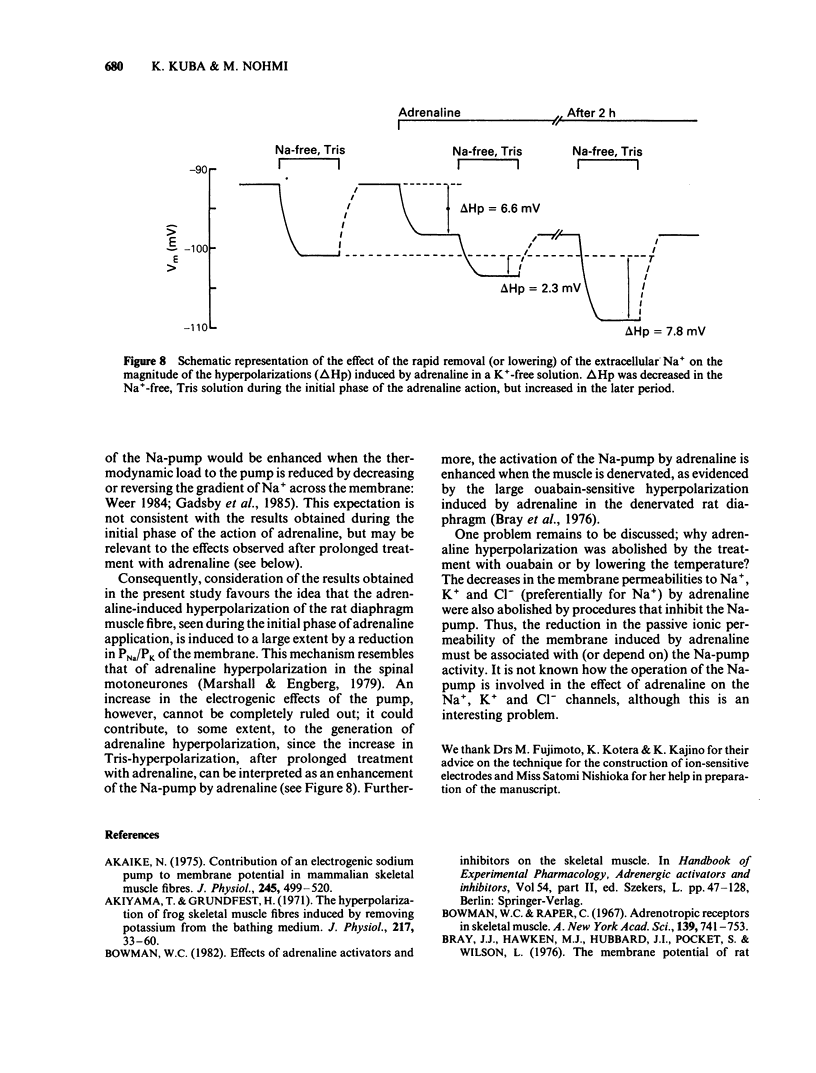
The ionic mechanism of membrane hyperpolarization induced by adrenaline in rat diaphragm muscle fibres was studied. Removal of the extracellular K+ ([K+]o) from Krebs-Ringer solution initially increased the resting membrane potential and then caused an increase in the intracellular Na+ activity ([Na+]i) and a decrease in the intracellular K+ activity ([K+]i). All the changes were maintained for more than 3 h. Application of ouabain (0.1 mM) or lowering the temperature rapidly reduced the resting potential by about 10 mV in the K+-free solution. It then produced further progressive decreases in resting potential and in [K+]i and a progressive increase in [Na+]i. These observations indicate that an electrogenic Na-pump operates in the K+-free solution. Removal of most of the Cl- in the K+-free solution did not affect the resting potential or the magnitude of the initial decrease produced by ouabain, despite an increased input resistance; this result implies a passive distribution of Cl-. Adrenaline (30-60 microM) either added to the bathing solution or applied to the membrane by ionophoresis produced a hyperpolarization (3-10 mV: adrenaline hyperpolarization), the amplitude of which was decreased with a rise in [K+]o and increased with a reduction in [K+]o, but unaffected by the removal of Cl-. Adrenaline produced an increase in input resistance, the relative magnitude (17-18%) of which was constant whether external K+ or Cl- was removed. In contrast, a conditioning membrane hyperpolarization hardly affected the resistance. Ouabain (0.1 mM) or low temperature (8-10 degrees C) abolished both the hyperpolarization and the increased input resistance induced by adrenaline. The [K+]i, [Na+]i and the peak of the action potential remained unchanged after a 20 min exposure to adrenaline (30 microM). The hyperpolarization induced by the replacement of all Na+ with Tris (Tris-hyperpolarization) in the K+-free solution was depressed by 39% during the early period (4-31 min) of exposure to adrenaline (30 microM), while it was enhanced by 26% during the later period (80-130 min). The initial depression suggested a decrease in the ratio of the membrane permeability for Na+ (PNa) to that for K+ (PK). These results suggest that the adrenaline hyperpolarization is generated largely by a decrease in PNa/PK, which is associated with the activity of the Na-pump.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N. Contribution of an electrogenic sodium pump to membrane potential in mammalian skeletal muscle fibres. J Physiol. 1975 Mar;245(3):499–520. doi: 10.1113/jphysiol.1975.sp010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Grundfest H. The hyperpolarization of frog skeletal muscle fibres induced by removing potassium from the bathing medium. J Physiol. 1971 Aug;217(1):33–60. doi: 10.1113/jphysiol.1971.sp009558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Raper C. Adrenotropic receptors in skeletal muscle. Ann N Y Acad Sci. 1967 Feb 10;139(3):741–753. doi: 10.1111/j.1749-6632.1967.tb41241.x. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Hawken M. J., Hubbard J. I., Pockett S., Wilson L. The membrane potential of rat diaphragm muscle fibres and the effect of denervation. J Physiol. 1976 Mar;255(3):651–667. doi: 10.1113/jphysiol.1976.sp011301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler B. H., Phillis J. W., Kozachuk W. Noradrenaline stimulation of a membrane pump in frog skeletal muscle. Eur J Pharmacol. 1975 Aug;33(1):201–204. doi: 10.1016/0014-2999(75)90158-2. [DOI] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977 Sep;270(2):383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A., Mooij J. J. Potassium induced potential changes in rat diaphragm muscle. Pflugers Arch. 1976 Mar 11;362(1):69–79. doi: 10.1007/BF00588683. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J Physiol. 1978 Mar;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Kubota T. Physicochemical properties of a liquid ion exchanger microelectrode and its application to biological fluids. Jpn J Physiol. 1976;26(6):631–650. doi: 10.2170/jjphysiol.26.631. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- Kaibara K., Koketsu K., Akasu T., Miyagawa M. A kinetic analysis of the facilitatory action of adrenaline. Pflugers Arch. 1982 Jan;392(3):304–306. doi: 10.1007/BF00584316. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Ohta Y. Acceleration of the electrogenic Na+ pump by adrenaline in frog skeletal muscle fibres. Life Sci. 1976 Oct 1;19(7):1009–1013. doi: 10.1016/0024-3205(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Koketsu K. The electrogenic sodium pump. Adv Biophys. 1971;2:77–112. [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Kuba M., Koketsu K. Adrenaline hyperpolarization in rat diaphragm muscle fibers. Nihon Seirigaku Zasshi. 1978 Oct;40(10):377–380. [PubMed] [Google Scholar]
- Marshall K. C., Engberg I. Reversal potential for noradrenaline-induced hyperpolarization of spinal motoneurons. Science. 1979 Jul 27;205(4404):422–424. doi: 10.1126/science.451613. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. Catecholamines and the sodium pump in excitable cells. Prog Neurobiol. 1981;17(3):141–184. doi: 10.1016/0301-0082(81)90012-5. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A. Contributions of electrogenic pumps to resting membrane potentials: the theory of electrogenic potentials. Soc Gen Physiol Ser. 1984;38:105–127. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Pharmacology of excitation-contraction coupling in vascular smooth muscle and in avian slow muscle. Fed Proc. 1969 Sep-Oct;28(5):1634–1642. [PubMed] [Google Scholar]
- Tashiro N. Effects of isoprenaline on contractions of directly stimulated fast and slow skeletal muscles of the guinea-pig. Br J Pharmacol. 1973 May;48(1):121–131. doi: 10.1111/j.1476-5381.1973.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]



