Abstract
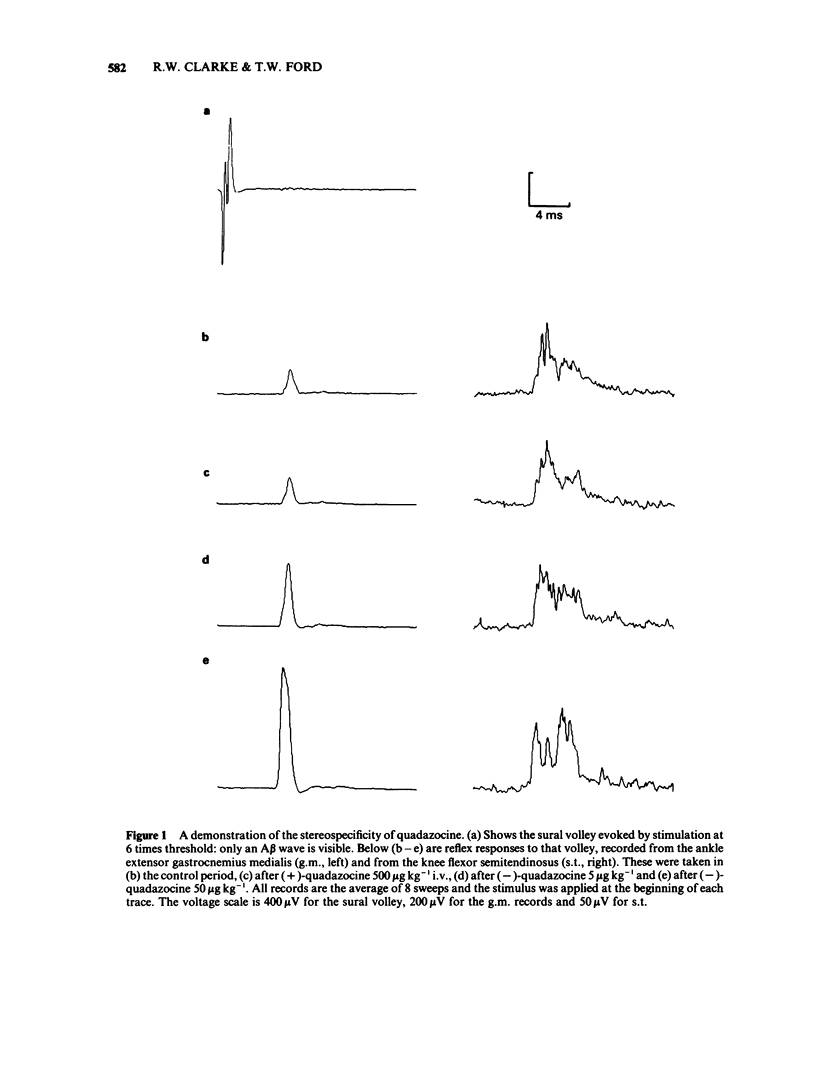
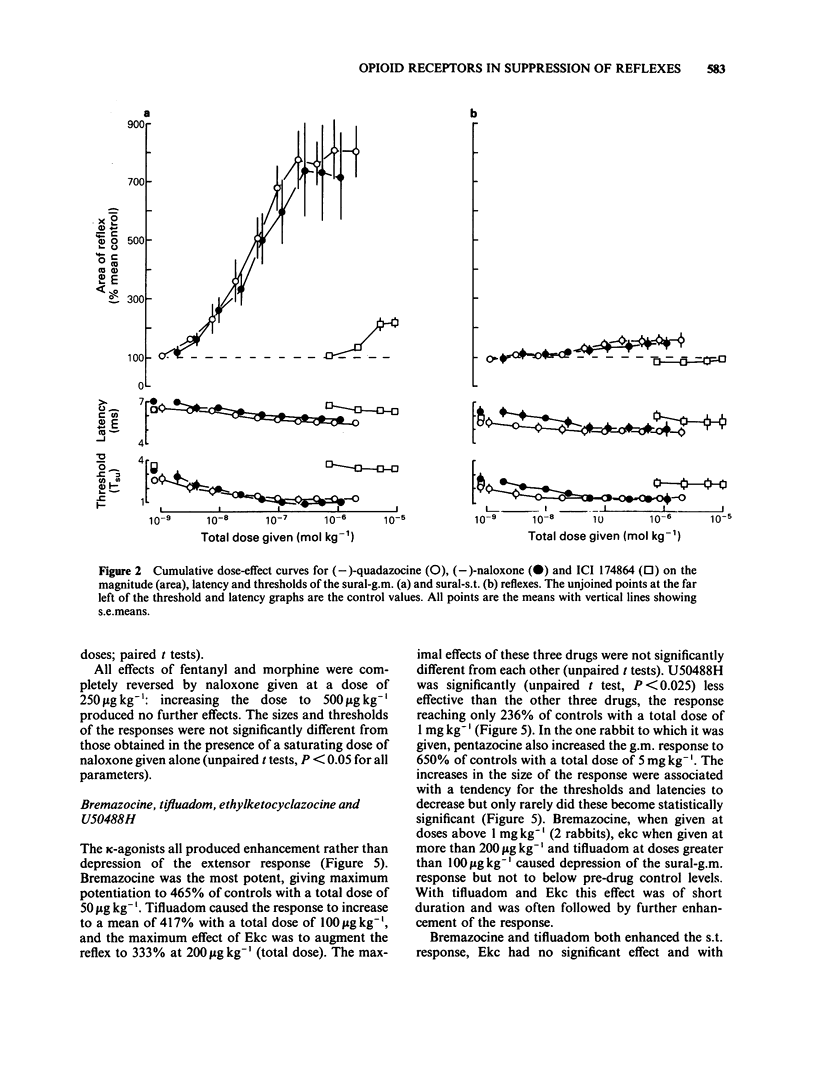
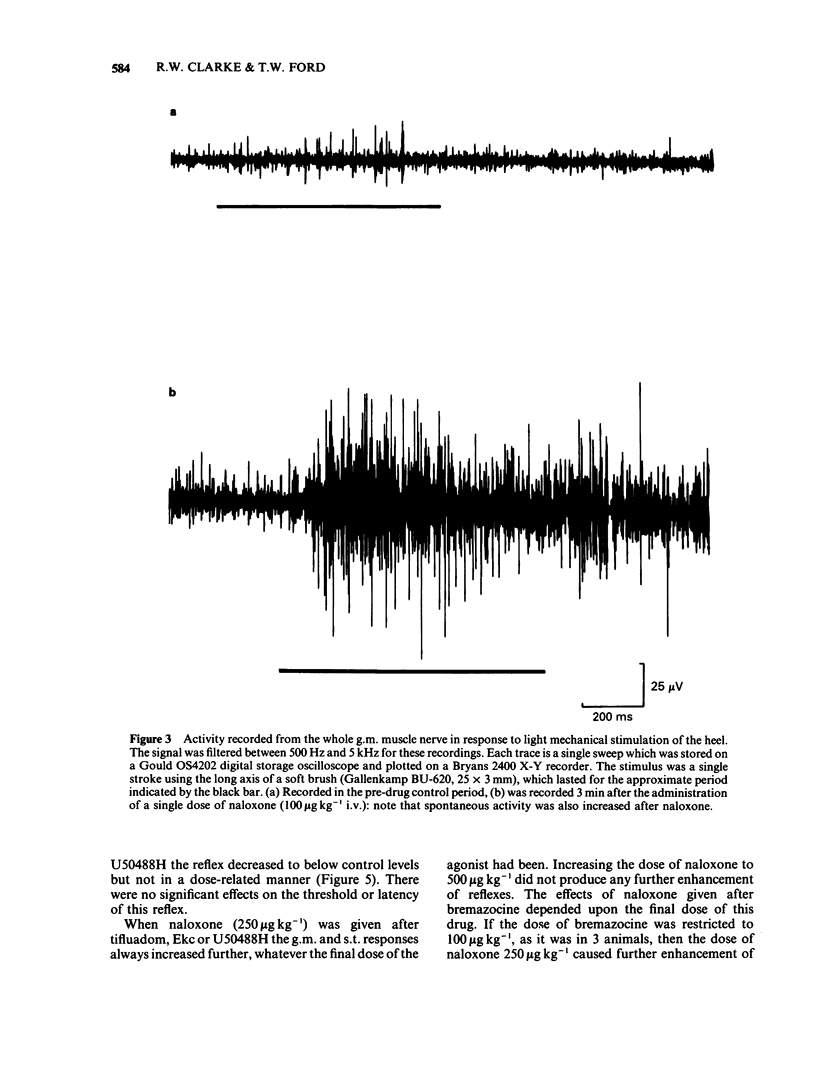
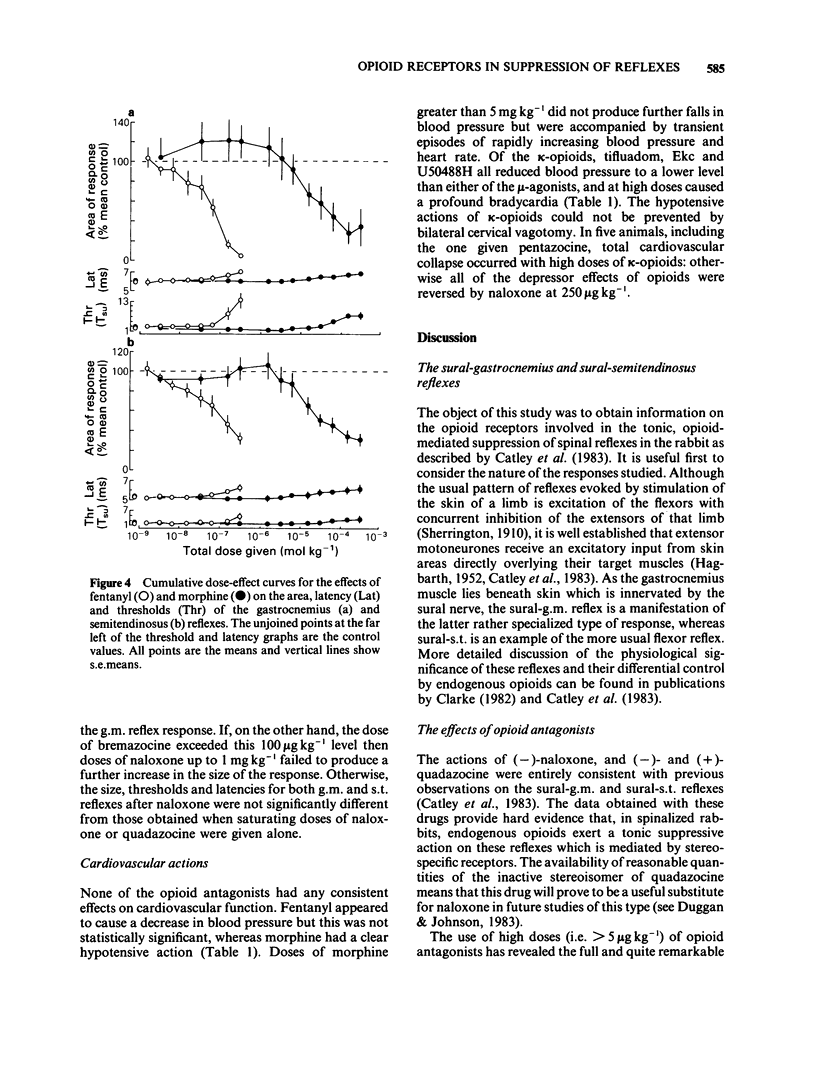
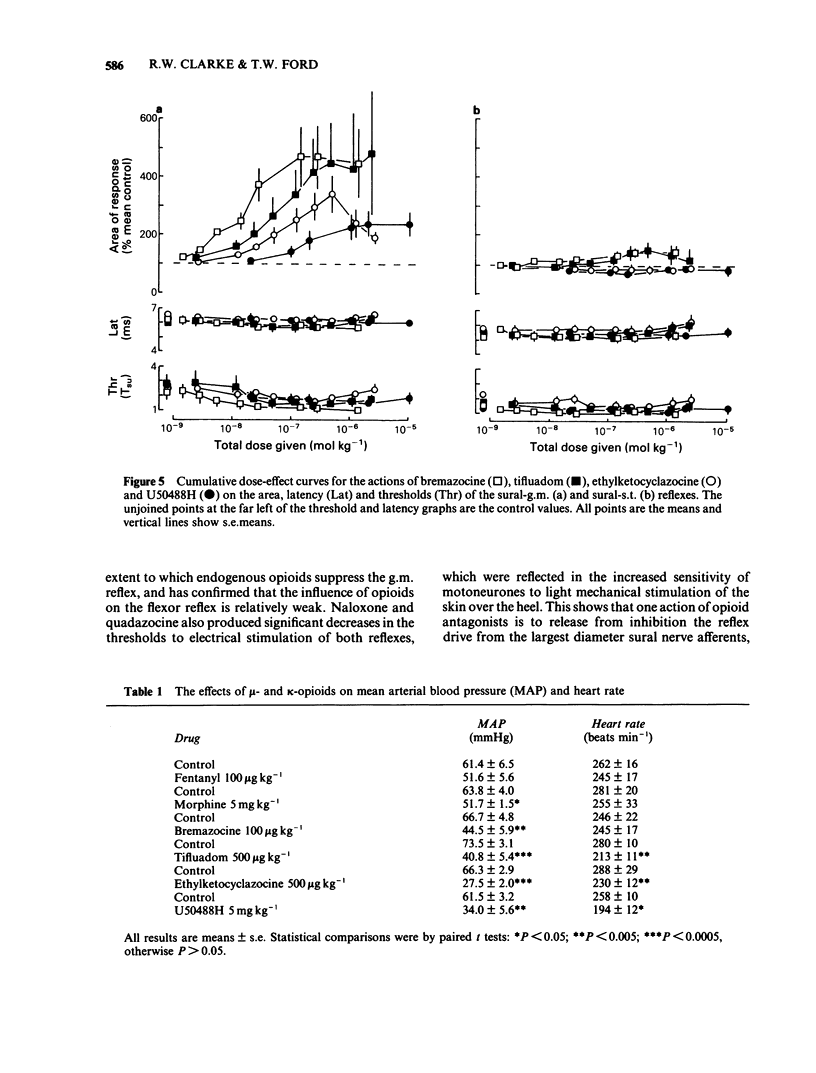
Spinal reflexes in the rabbit are suppressed tonically by endogenous opioids. The contributions made to this suppression by mu-, delta- and kappa-opioid receptors have been investigated by studying the actions of a range of opioid antagonists and agonists on reflexes evoked by sural nerve stimulation in the ankle extensor gastrocnemius medialis (g.m.), and in the knee flexor semitendinosus (s.t.). When given at a total dose of 88.5 micrograms kg-1 i.v., either of the universal opioid receptor antagonists (-)-naloxone and (-)-quadazocine enhanced the g.m. response to more than 7 times the pre-drug control values, and the s.t. reflex to 1.5 times controls. The effects of quadazocine were stereospecific. The selective delta antagonist ICI 174864 (3.5 mg kg-1 i.v. total) also augmented the g.m. reflex but only to twice pre-drug controls. The mu-agonists fentanyl (100 micrograms kg-1) and morphine (50 mg kg-1) suppressed both g.m. and s.t. reflex responses to less than half control levels by a naloxone-reversible mechanism. The kappa-agonists bremazocine (50 micrograms kg-1 total), tifluadom (100 micrograms kg-1), ethylketocyclazocine (200 micrograms kg-1) and U50488H (1 mg kg-1) potentiated the g.m. reflex and had variable effects on the s.t. response. Naloxone usually added to the facilitatory actions of these drugs. kappa-Opioid receptor agonists also caused a profound, naloxone-reversible depression of arterial blood pressure. It may be concluded that the endogenous opioid-mediated suppression of spinal reflexes in the rabbit is mediated mainly, if not exclusively, through mu-receptors. There are no known endogenous ligands which are specific for the mu-receptor, so in the present case it seems that selectivity is determined by the receptor population rather than by the ligand.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmans N., Carroll J. A., Rance M. J., Simon E. J., Traynor J. R. Sodium ions increase the binding of the antagonist peptide ICI 174864 to the delta-opiate receptor. Neuropeptides. 1986 Feb-Mar;7(2):139–143. doi: 10.1016/0143-4179(86)90089-2. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Martin W. R. The effect of the narcotic antagonists naloxone, naltrexone and nalorphine on spinal cord C-fiber reflexes evoked by electrical stimulation or radiant heat. Eur J Pharmacol. 1977 Mar 21;42(2):147–154. doi: 10.1016/0014-2999(77)90354-5. [DOI] [PubMed] [Google Scholar]
- Catley D. M., Clarke R. W., Pascoe J. E. Naloxone enhancement of spinal reflexes in the rabbit. J Physiol. 1983 Jun;339:61–73. doi: 10.1113/jphysiol.1983.sp014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hall J. G., Headley P. M. Enkephalins and dorsal horn neurones of the cat: effects on responses to noxious and innocuous skin stimuli. Br J Pharmacol. 1977 Nov;61(3):399–408. doi: 10.1111/j.1476-5381.1977.tb08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Morton C. R., Johnson S. M., Zhao Z. Q. Opioid antagonists and spinal reflexes in the anaesthetized cat. Brain Res. 1984 Apr 9;297(1):33–40. doi: 10.1016/0006-8993(84)90540-7. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., North R. A. Electrophysiology of opioids. Pharmacol Rev. 1983 Dec;35(4):219–281. [PubMed] [Google Scholar]
- Duggan A. W., Zhao Z. Q. Microelectrophoretic administration of naloxone near motoneurones fails to reproduce the effects of systemic naloxone in anaesthetized cats. Neurosci Lett. 1984 Apr 6;45(3):305–310. doi: 10.1016/0304-3940(84)90243-x. [DOI] [PubMed] [Google Scholar]
- Ensinger H., Hedler L., Schurr C., Starke K. Ethylketocyclazocine decreases noradrenaline release and blood pressure in the rabbit at a peripheral opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 1984 Nov;328(1):20–23. doi: 10.1007/BF00496099. [DOI] [PubMed] [Google Scholar]
- Ensinger H., Hedler L., Szabo B., Starke K. Bremazocine causes sympatho-inhibition and hypotension in rabbits by activating peripheral kappa-receptors. J Cardiovasc Pharmacol. 1986 May-Jun;8(3):470–475. doi: 10.1097/00005344-198605000-00005. [DOI] [PubMed] [Google Scholar]
- Goldfarb J., Hu J. W. Enhancement of reflexes by naloxone in spinal cats. Neuropharmacology. 1976 Dec;15(12):785–792. doi: 10.1016/0028-3908(76)90009-5. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Excitatory and inhibitory skin areas for flexor and extensor motoneurons. Acta Physiol Scand Suppl. 1952;26(94):1–58. [PubMed] [Google Scholar]
- Hayes A. G., Skingle M., Tyers M. B. Reversal by beta-funaltrexamine of the antinociceptive effect of opioid agonists in the rat. Br J Pharmacol. 1986 Aug;88(4):867–872. doi: 10.1111/j.1476-5381.1986.tb16260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley P. M., Parsons C. G., West D. C. Comparison of mu, kappa and sigma preferring agonists for effects on spinal nociceptive and other responses in rats. Neuropeptides. 1984 Dec;5(1-3):249–252. doi: 10.1016/0143-4179(84)90074-x. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Porreca F., Burks T. F. Mu, but not kappa, opioid agonists induce contractions of the canine small intestine ex vivo. Eur J Pharmacol. 1985 Feb 12;109(1):49–54. doi: 10.1016/0014-2999(85)90538-2. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W. The Wellcome Foundation lecture, 1982. Opioid peptides and their receptors. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):27–40. doi: 10.1098/rspb.1985.0048. [DOI] [PubMed] [Google Scholar]
- Magnan J., Paterson S. J., Tavani A., Kosterlitz H. W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- McClane T. K., Martin W. R. Effects of morphine, nalorphine, cyclazocine, and naloxone on the flexor reflex. Int J Neuropharmacol. 1967 Mar;6(2):89–98. doi: 10.1016/0028-3908(67)90057-3. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Kouakou Y., Puget A., Moisand C. Multiple opiate binding sites in the central nervous system of the rabbit. Large predominance of a mu subtype in the cerebellum and characterization of a kappa subtype in the thalamus. Mol Pharmacol. 1983 Jul;24(1):23–29. [PubMed] [Google Scholar]
- Miller L., Shaw J. S., Whiting E. M. The contribution of intrinsic activity to the action of opioids in vitro. Br J Pharmacol. 1986 Mar;87(3):595–601. doi: 10.1111/j.1476-5381.1986.tb10202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Negishi K., Suda M., Matsumiya T., Inazu T., Ueki M. Rabbit vas deferens: a specific bioassay for opioid kappa-receptor agonists. Eur J Pharmacol. 1981 Jul 17;73(2-3):235–236. doi: 10.1016/0014-2999(81)90098-4. [DOI] [PubMed] [Google Scholar]
- Roppolo J. R., Booth A. M., De Groat W. C. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res. 1983 Apr 4;264(2):355–358. doi: 10.1016/0006-8993(83)90841-7. [DOI] [PubMed] [Google Scholar]
- Römer D., Büscher H. H., Hill R. C., Maurer R., Petcher T. J., Zeugner H., Benson W., Finner E., Milkowski W., Thies P. W. An opioid benzodiazepine. Nature. 1982 Aug 19;298(5876):759–760. doi: 10.1038/298759a0. [DOI] [PubMed] [Google Scholar]
- Römer D., Büscher H., Hill R. C., Maurer R., Petcher T. J., Welle H. B., Bakel H. C., Akkerman A. M. Bremazocine: a potent, long-acting opiate kappa-agonist. Life Sci. 1980 Sep 15;27(11):971–978. doi: 10.1016/0024-3205(80)90107-1. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. F., Rance M. J. Opiate receptors in the rat vas deferens. Life Sci. 1983;33 (Suppl 1):327–330. doi: 10.1016/0024-3205(83)90509-x. [DOI] [PubMed] [Google Scholar]
- Struppler A., Burgmayer B., Ochs G. B., Pfeiffer H. G. The effect of epidural application of opioids on spasticity of spinal origin. Life Sci. 1983;33 (Suppl 1):607–610. doi: 10.1016/0024-3205(83)90576-3. [DOI] [PubMed] [Google Scholar]
- Szabo B., Hedler L., Ensinger H., Starke K. Opioid peptides decrease noradrenaline release and blood pressure in the rabbit at peripheral receptors. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):50–56. doi: 10.1007/BF00633196. [DOI] [PubMed] [Google Scholar]
- Vonvoigtlander P. F., Lahti R. A., Ludens J. H. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983 Jan;224(1):7–12. [PubMed] [Google Scholar]
- Ward S. J., Pierson A. K., Michne W. F. Multiple opioid receptor profile in vitro and activity in vivo of the potent opioid antagonist Win 44,441-3. Life Sci. 1983;33 (Suppl 1):303–306. doi: 10.1016/0024-3205(83)90503-9. [DOI] [PubMed] [Google Scholar]


