Abstract
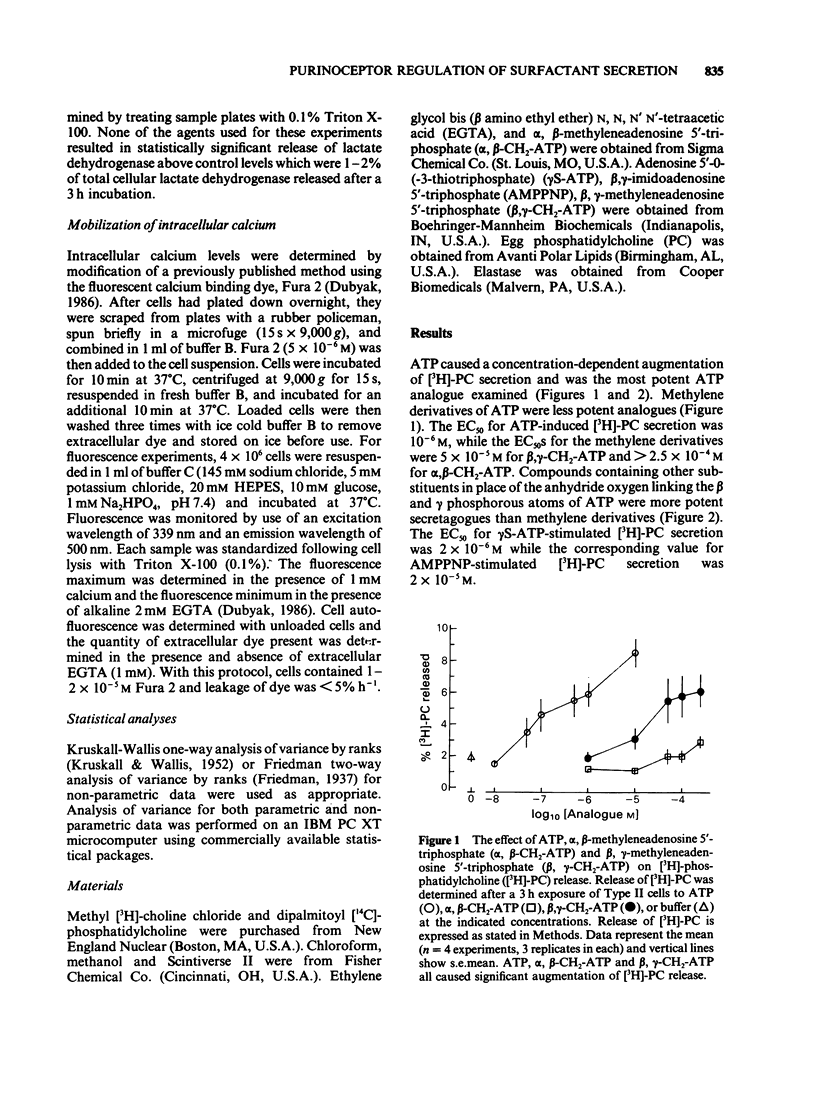
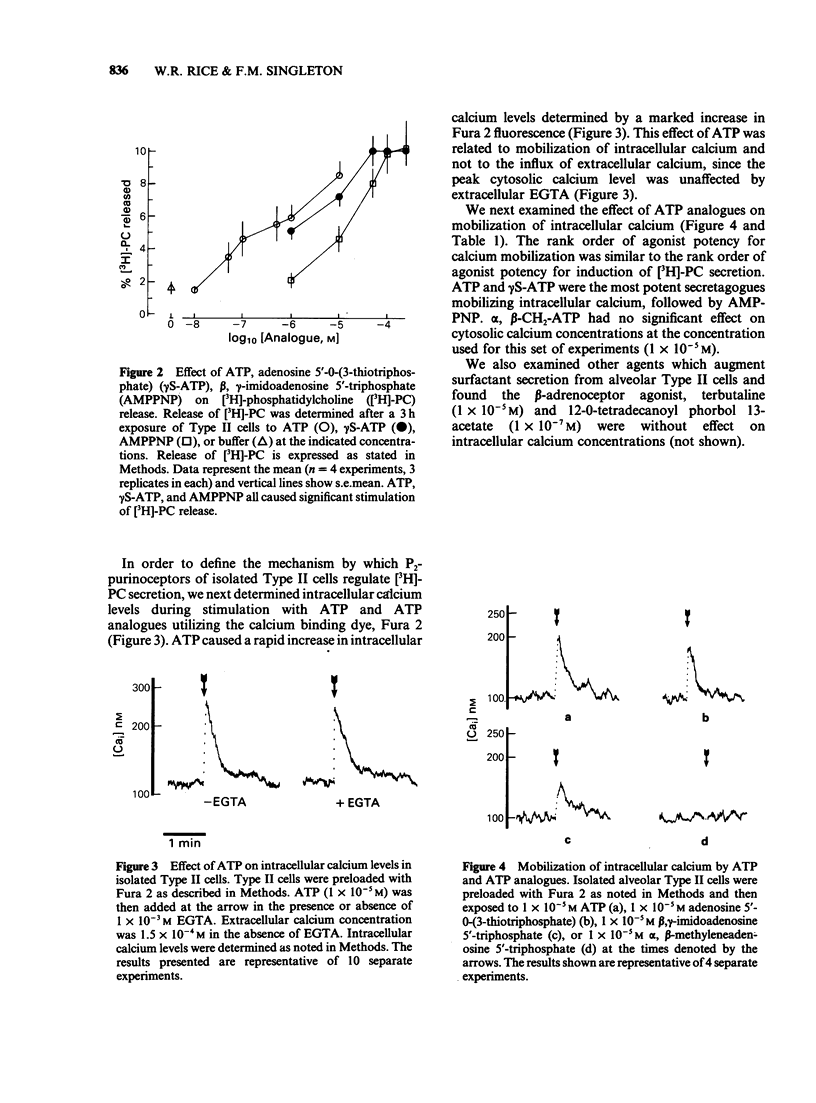
1 The effect of methylene, thio, and imido substituted analogues of adenosine 5'-triphosphate (ATP) on surfactant phospholipid secretion and calcium mobilization in rat isolated alveolar Type II cells was studied. 2 ATP was the most potent secretagogue of adenine nucleotides studied. The rank order of agonist potency for [3H]-phosphatidylcholine secretion was ATP greater than adenosine 5'-O-(3-thiotriphosphate) (gamma S-ATP) greater than beta, gamma-imido adenosine 5'-triphosphate (AMPPNP) greater than beta, gamma-methylene adenosine 5'-triphosphate (beta, gamma-CH2-ATP) greater than alpha, beta-methylene adenosine 5'-triphosphate (alpha, beta-CH2-ATP). The respective EC50S were 10(-6) M, 2 X 10(-6) M, 2 X 10(-5) M, and greater than 2.5 X 10(-4) M. 3 Exogenous ATP also induced a rapid mobilization of intracellular calcium monitored by changes in Fura 2 fluorescence. The rank order of agonist potency for calcium mobilization was similar to the rank order of agonist potency for surfactant secretion: ATP = gamma S-ATP greater than AMPPNP greater than alpha, beta-CH2-ATP. 4 There was no effect of EGTA on ATP-induced calcium mobilization, consistent with the hypothesis that exogenous ATP induces release of calcium from intracellular stores. 5 These data are consistent with a P2Y-purinoceptor regulating surfactant secretion from isolated Type II cells via mobilization of intracellular calcium, since: (a) non-hydrolyzed analogues of ATP are potent secretagogues, (b) beta, gamma-CH2-ATP was a more potent secretagogue than alpha, beta-CH2-ATP and (c) the rank orders of agonist potency for calcium mobilization and phospholipid secretion were the same.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Effects of phosphate-modified adenine nucleotide analogues on insulin secretion from perfused rat pancreas. Br J Pharmacol. 1981 May;73(1):105–110. doi: 10.1111/j.1476-5381.1981.tb16778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R., Williams M. C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986 Jul;134(1):141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M., Rooney S. A. Purinoceptor agonists stimulate phosphatidylcholine secretion in primary cultures of adult rat type II pneumocytes. Biochim Biophys Acta. 1987 Jan 13;917(1):18–23. doi: 10.1016/0005-2760(87)90278-5. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARP N. A. The release of catechol amines from the amine containing granules of the adrenal medulla. Acta Physiol Scand. 1958 Oct 8;43(3-4):292–302. doi: 10.1111/j.1748-1716.1958.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M., Gilfillan A. M. The pharmacology of lung surfactant secretion. Pharmacol Rev. 1984 Jun;36(2):69–90. [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Rooney S. A. Surfactant secretion in a newborn rabbit lung slice model. Biochim Biophys Acta. 1980 Dec 5;620(3):509–519. doi: 10.1016/0005-2760(80)90143-5. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Dobbs L. G., Greenleaf R. D., Williams M. C. Alveolar type II cells. Fed Proc. 1977 Dec;36(13):2697–2702. [PubMed] [Google Scholar]
- Oyarzún M. J., Clements J. A. Ventilatory and cholinergic control of pulmonary surfactant in the rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):39–45. doi: 10.1152/jappl.1977.43.1.39. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Hull W. M., Dion C. A., Hollinger B. A., Whitsett J. A. Activation of cAMP dependent protein kinase during surfactant release from type II pneumocytes. Exp Lung Res. 1985;9(1-2):135–149. doi: 10.3109/01902148509061533. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Singleton F. M. P2-purinoceptors regulate surfactant secretion from rat isolated alveolar type II cells. Br J Pharmacol. 1986 Nov;89(3):485–491. doi: 10.1111/j.1476-5381.1986.tb11148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Voelker D. R., Mason R. J. Involvement of protein kinase C in pulmonary surfactant secretion from alveolar type II cells. J Biol Chem. 1985 Oct 15;260(23):12725–12729. [PubMed] [Google Scholar]


