Abstract
Estrogen induces both rapid and delayed effects on the cardiovascular system. The early effects take place within minutes (e.g., changes in vasomotor tone) and are mediated through rapid intracellular signaling pathways; whereas the delayed effects (e.g., remodeling or lipid alterations) require hours to days to occur and require transcriptional effects with subsequent modulation of protein expression. To study the acute effects of 17β-estradiol (E2) treatment on vascular function, we have investigated the rapid (on the order of minutes) effects of E2 treatment on intracellular signaling in human endothelial cells (EC). Our previous data have shown that E2 induces rapid release of NO from and activation of guanylate cyclase in human EC. In this study, we demonstrate that E2 also activates mitogen-activated protein kinase (extracellular signal-related kinase) signaling within minutes in EC. We hypothesized that this effect might be mediated by estrogen receptors (ER) localized to the cell surface. Our data show that membrane-impermeant forms of E2 also activate EC mitogen-activated protein kinase as well as stimulate cGMP production and NO release. The ER antagonist ICI 182,780 blocks this effect. Using confocal microscopy and flow cytometric analysis, we demonstrate that EC contain surface binding sites for E2, detectable by cell-impermeant ligand binding and equally with an anti-ERα antibody. Immunoreactive bands of 66 and 45 kDa are detectable with an anti-ERα mAb in human EC, and their individual presence correlates functionally with E2-stimulated genomic and rapid nongenomic responses, respectively. Membrane ERs may provide key molecular switches in these novel, rapid signaling pathways induced by E2 in EC.
Estrogens have a wide array of biological effects. The cell type and tissue specificity of estrogens allows for a single molecule to have both agonist and antagonist activity within the same organism. The ability of estrogens to target both genomic and nongenomic mechanisms leads to activation of signaling pathways which may occur within minutes or require hours in a single cell type. For example, the effects of estrogens on the human cardiovascular system include long-term alterations in lipid profiles, requiring days to weeks to occur, as well as rapid vasodilation, occurring within minutes of estradiol administration. This diversity of effects can only partially be explained by our current understanding of estrogen receptor (ER) structure and function. Ligand binding of ERs leads to direct structural modifications of the receptor, such as changes in phosphorylation and alterations in the proteins bound to the receptor. Furthermore, the cytoplasmic vs. nuclear localization of receptors also appears to be affected by ligand binding.
These previous ER models are most useful for understanding the slower, genomic signaling pathways by which estrogens affect cellular functions. More recently, we and others have described rapid signaling effects of estrogens, which are poorly explained by these models. For example, estrogen stimulation of human umbilical vein endothelial cells (HUVEC) results in release of NO and increases in intracellular cGMP which occur within minutes of hormone addition (1). As predicted by their time course, these effects are independent of transcription, and thus, do not fit the classic concept of nuclear localization and genomic regulation by estrogen-bound ER. However, activation of estrogen-induced signaling pathways can be blocked by the same synthetic ER antagonists that block transcriptional activation by the classically described ERs (α and β). This suggests that the receptor molecule responsible for rapid cellular responses to estrogen is similar, if not identical, to that responsible for the genomic effects of estrogen.
Acknowledging the nongenomic effects of steroid hormones in other cell types has led several investigators to correctly hypothesize that cell surface forms of these receptors exist and participate in activation of signaling pathways previously thought to be restricted to transmembrane peptide hormone receptor-signaling molecules. For example, cell surface receptors for progesterone have been identified in a number of cell types and have been shown to mediate rapid alterations in intracellular calcium (2). This line of investigation has broadened our understanding of the mechanisms by which steroid hormones produce such varied physiologic effects.
The presence of binding sites for estrogen on cell surface membranes has been a topic of debate for many years (3). Such binding sites were demonstrated by intact cell binding to estradiol immobilized on nylon fibers over 20 years ago and were believed to participate in cellular uptake of estrogen for delivery to intracellular target receptors (4). However, the role of these surface receptors in signaling has not been explored until recently. Chinese hamster ovary cells transiently transfected with human expression constructs have been shown to express a small amount of ER protein on their surface and to respond to estrogen with rapid generation of inositol phosphate (5).
We have demonstrated that in addition to rapid release of NO, estrogen stimulation of human endothelial cells (EC) leads to activation of the mitogen-activated protein kinase (MAPK) pathway within minutes. Herein, we demonstrate that cell-impermeant estrogens rapidly trigger multiple key signaling cascades and that plasma membrane estrogen binding sites are present on HUVEC. We discuss the importance of these hormone-activated rapid responses in vascular endothelium.
Materials and Methods
Cell Culture and Reagents.
HUVEC were isolated from single donors as described previously (6). Cells were routinely passaged on gelatin-coated plates in M199 with 15% FBS, bovine endothelial cell growth supplement (ECGS) (50 μg/ml), and heparin (100 μg/ml). The permanently established EA.hy926 endothelial cell line (7) was generously provided by C. J. S. Edgell (University of North Carolina). Cells were routinely passaged in DMEM with 10% FBS, hypoxanthine (5 mM), thymidine (0.8 mM), and aminopterin (20 μM). Before estrogen stimulation, cells were cultured in phenol red-free M199 or DMEM with gelding horse serum (lot 114H4629, <1.0 pg/ml estradiol; Sigma) and no ECGS/heparin for 24–48 h. All estrogen species [17β-estradiol (E2), β-estradiol-6-(O-carboxymethyl)oxime:BSA (E2coBSA, ≈30 mol E2:mol BSA), and β-estradiol 17-hemisuccinate:BSA (E2hsBSA, ≈38 mol E2:mol BSA)] were purchased from Sigma.
MAPK Assays.
To reduce background MAPK activity, HUVEC were cultured in phenol red-free media with 5% BSA and no serum overnight. MAPK kinase activity was assessed by immunoprecipitation of activated extracellular signal-related kinase (ERK) 1/ERK2 (p42 and p44 MAPK, using a phospho-specific antibody) and a subsequent in vitro enzymatic assay using an ELK1 substrate peptide. Phosphorylated ELK1 was then detected by immunoblotting using a phospho-specific anti-ELK1 antibody (New England BioLabs).
cGMP Assays.
cGMP was acid extracted from intact cell monolayers as described previously (8). Samples were pH neutralized and cGMP levels were measured using an RIA kit (Biomedical Technologies, Stoughton, MA). ICI 182,780 was purchased from Zeneca (Wilmington, DE).
NO Release Assays.
NO-specific chemiluminescence was used to determine the amount of NO2−, NO3−, and nitrothiols (cumulatively referred to as NOx) released from EA.hy926 cell monolayers, as previously described (1). Cells were switched to E2-free medium for 48 h before stimulation with E2coBSA for 30 min at 37°C, after which the monolayers were washed and the medium was changed to Hanks' balanced salt solution supplemented with CaCl2 (1.2 mmol/liter), MgSO4 (0.6 mmol/liter), and l-arginine (100 μmol/liter). Some cells were treated with ICI 182,780 for 1 h before stimulation. The medium was equilibrated for 1 h at 37°C, after which supernatants were collected for NOx analysis as previously described (1).
Immunofluorescence.
HUVEC were grown in monolayers on fibronectin-coated (2 μg/cm2; Collaborative Biomedical Products, Bedford, MA) glass slides in 12-well plates. Monolayers of cells were stained on ice or after fixation with acetone on ice. All blocking and incubation steps were performed in the presence of 5% BSA. E2coBSA covalently linked to FITC (Sigma) was resuspended in PBS at a concentration of 1 mg/ml before use. Confocal microscopy was carried out using a Bio-Rad MRC confocal system with a Zeiss Axiovert microscope.
Fluorescence-Activated Cell Sorter (FACS).
Single-cell suspensions of HUVEC and EA.hy926 cells were obtained from monolayers using trypsin/EDTA or nonenzymatic chelating solution (cell dissociation solution; Sigma). Cells were stained on ice in the presence of 5% BSA. Single and dual-color FACS analyses were carried out on the FACSort system using Lysis II software (Becton Dickinson). The anti-estrogen receptor antibody 1D5 was obtained from Dako. Secondary goat anti-mouse IgG (GAM) conjugated to phycoerythrin (PE) was purchased from Sigma. E2coBSA covalently coupled to FITC (E2coBSA-FITC; Sigma) was resuspended at 1 mg/ml in PBS. In some experiments, cells were treated on ice with ICI 182,780 or a 20-fold excess of E2 for 1h before E2coBSA-FITC staining.
Transfections.
EA.hy926 cells were grown to 70% confluence and transiently transfected with 1 μg of plasmid DNA using LipofectAMINE and LipofectAMINE PLUS reagent according to the manufacturer's specifications (Life Technologies, Grand Island, NY). The plasmid constructs used have been described previously (9, 10). hERα0Pu encoding full-length ERα was kindly provided by P. Chambon (Université Louis Pasteur, Paris, France) and the reporter construct pGL3-pro-3(EREc38)-luciferase was a generous gift from C. Klinge (University of Louisville, Louisville, KY). Cytomegalovirus-β-galactosidase (CLONTECH) was used for normalization. ERα was detected in non- and transiently-transfected EA.hy926 cells and HUVEC by Western blotting and enhanced chemiluminescence using monoclonal ERα antibody, F-10 (Santa Cruz Biotechnology), which recognizes a C-terminal epitope.
Results
Estrogen Stimulation of HUVEC Leads to Rapid Activation of MAPK.
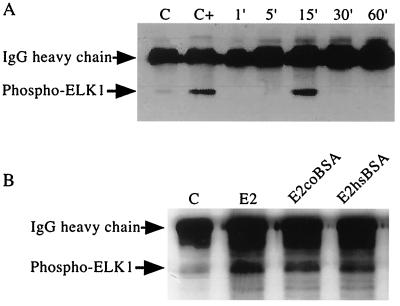
We have previously shown that estradiol stimulation of HUVEC leads to rapid increases in NO release that are accompanied by activation of guanylate cyclase and cGMP production. More recently, we have observed that these effects of estradiol are blocked in the presence of the tyrosine kinase inhibitor, herbimycin (11). Furthermore, rapid activation of tyrosine phosphorylation and MAPK activity by estradiol has been reported in breast, bone, and neuronal cells lines (12–14). Based on these findings, we investigated whether signaling pathways, which are downstream of common receptor tyrosine kinases, are rapidly activated by estradiol. To test whether the MAPK signaling pathway is involved, we stimulated HUVEC for various periods of time with E2, immunoprecipitated activated MAPK (phospho-ERK1 and ERK2), and measured its enzymatic activity in an in vitro kinase assay with an ELK-1 peptide substrate. Fig. 1A shows that these MAPKs are activated within 15 min of E2 addition to HUVEC. This activity rapidly declines to basal levels within 1 h after stimulation. These data support the recent finding that E2 treatment of ovine pulmonary artery EC increases MAPK activity within 5 min of treatment and further demonstrates the transient nature of this signal in human EC (15).
Figure 1.
HUVEC MAPK activation by free and membrane-impermeant forms of estrogen. Serum-deprived HUVEC monolayers were E2 (50 ng/ml)-treated for the indicated times (A) or treated for 15 min with either E2 (50 ng/ml), E2coBSA (395 ng/ml, equivalent to 50 ng/ml of E2), or E2hsBSA (308 ng/ml, equivalent to 50 ng/ml of E2) (B). Subsequently, activated ERK1/2, immunoprecipitated from cell lysates, was used in an in vitro kinase assay with ELK-1 peptide substrate. Kinase reaction samples were immunoblotted with a phospho-specific anti-ELK-1 antibody. Controls were treated with 5% BSA containing media alone (C) or the addition of purified activated MAPK to the kinase reaction (C+).
Rapid Activation of MAPKs Occurs in Response to Membrane-Impermeant Forms of E2.
To test whether the activation of MAPK by estradiol can be mediated by binding of estradiol to endothelial cell surface receptors, human EC were treated with two different forms of estradiol covalently linked to BSA. As shown in Fig. 1B, both E2coBSA and E2hsBSA stimulation of HUVEC leads to rapid stimulation of MAPK activity similar to that seen with free estradiol. Since cells were cultured overnight in 5% BSA and control cells were treated with BSA in parallel, it is unlikely that this activation is caused by the BSA moiety of these compounds. These findings suggest that interactions between E2 and EC surface sites can lead to activation of intracellular pathways which involve the MAPK signaling cascade.
These forms of macromolecular-bound estrogen are unable to enter cells and are much more water soluble than free E2 (16). To ensure the absence of free E2 in these preparations, aliquots were preabsorbed with dextran-coated charcoal under conditions previously shown to remove >99% of free steroid hormone (17). No difference in activity was found between non-charcoal treated and charcoal-treated aliquots (data not shown), suggesting that MAPK activation by these compounds is not mediated by significant contamination of free ligand.
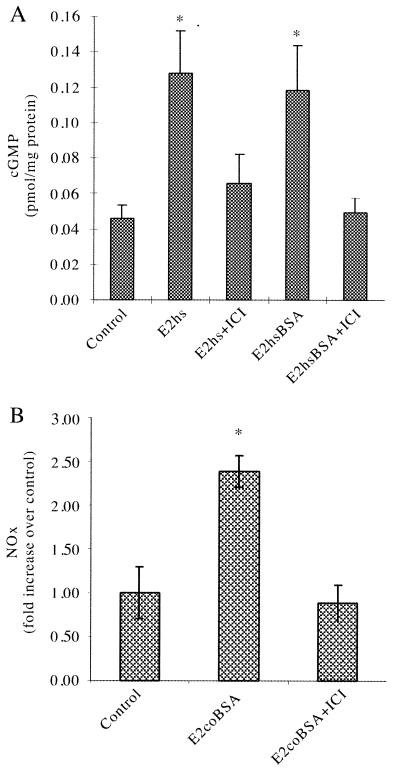
NO Release Is Rapidly Activated by Membrane-Impermeant E2 in an ER Antagonist-Inhibitable Manner.
We have previously demonstrated that estrogen stimulation of HUVEC leads to NO release and a consequent rapid increase in intracellular cGMP. These E2 effects on cGMP synthesis are mediated entirely by endothelial NO synthase-produced NO, in that they are blocked by the NO synthase competitive antagonist NG-nitro-L-arginine methyl ester (1). Fig. 2A shows that the addition of E2hsBSA leads to an ≈3-fold increase in intracellular cGMP similar to that seen with free E2hs. These effects are inhibited by the addition of the ER antagonist ICI 182,780, suggesting that the cell surface receptor which mediates these responses is similar to this ER. Fig. 2B displays similar findings of parallel NO release assays in which E2coBSA-stimulated EA.hy926 NO release is completely inhibited by ICI 182,780.
Figure 2.
cGMP and NOx production induced by membrane-impermeant E2. (A) HUVEC monolayers were treated with E2hs (equivalent to 10 ng/ml of E2) or E2hsBSA (equivalent to 10 ng/ml E2) in the presence or absence of the ER antagonist ICI 182,780 (10 μM) for 20 min at 37°C, after which cGMP levels were measured by RIA. (B) EA.hy926 monolayers were treated with E2coBSA (equivalent to 10 ng/ml of E2) in the absence or presence of ICI 182,780 for 30 min at 37°C, after which the amount of NOx released into the medium was determined by NO-specific chemiluminescence. *P < 0.01 vs. control.
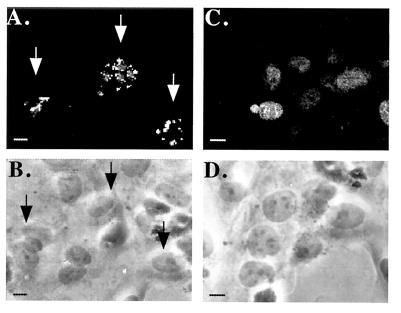
Human Endothelial Cells Express Cell Surface Binding Sites for Estradiol.
A small number of cell types have been described to express surface binding sites for estrogen. Exposure of intact cells to membrane-impermeant estrogens coupled to FITC results in a punctate staining pattern of the plasma membrane in these cell types (18–20). However, such binding sites have not been described on human vascular endothelial cells. Fig. 3A shows the staining pattern of nonfixed, nonpermeabilized HUVEC exposed to E2coBSA-FITC on ice. This punctate membrane staining pattern is remarkably similar to that previously described in both pituitary and breast tumor cell lines types (18, 20). Since cell labeling was carried out in excess free BSA, it is unlikely that the staining pattern observed is due to interaction with the BSA moiety of the molecule with the cell surface. Examination of multiple fields revealed that only a minority subset of HUVEC demonstrated this cell surface binding of E2coBSA-FITC (Fig. 3 A vs. B). However, identically prepared HUVEC, fixed and permeabilized, demonstrate typical nuclear E2 binding in a majority of cells (Fig. 3 C and D).
Figure 3.
Effect of EC permeabilization on immunofluorescent staining with membrane-impermeant estradiol. HUVEC monolayers grown on collagen-coated glass coverslips were stained on ice without fixation (A and B) or after fixation and permeabilization (C and D) by incubation with E2coBSA-FITC (1:100) in PBS with 5% BSA. Confocal immunofluorescence (A and C) or corresponding phase-contrast (B and D, respectively) micrographs are shown. Arrows denote intact cells staining with E2coBSA-FITC (A) and corresponding cells shown by phase microscopy (C). The photomicrographs shown are representative of multiple fields examined in three experiments (bar, 2.5 μM).
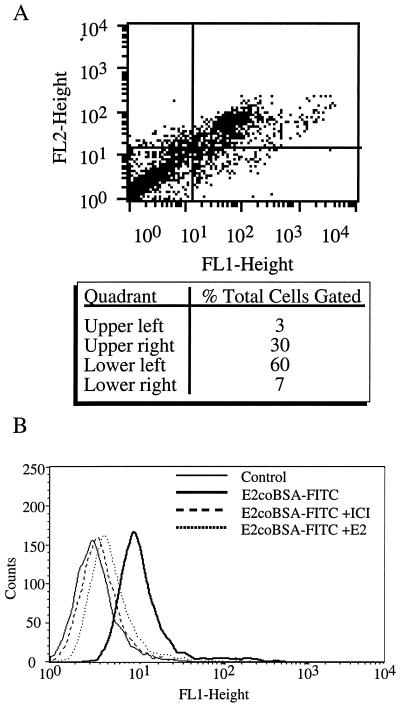
These results are supported by FACS analyses of nonpermeabilized HUVEC stained with E2coBSA-FITC on ice. In multiple preparations of single-donor HUVEC, a range from 1 to 30% of cells displayed surface binding sites for E2. Fig. 4A demonstrates a two-color FACS analysis of HUVEC stained with E2coBSA-FITC and mAb1D5 (plus secondary GAM-PE). The 1D5 recognition epitope is within the N terminus of ERα. This FACS profile demonstrates that the E2coBSA-positive EC are also 1D5 positive, and that this population represents about 30% of HUVEC in the example shown. These findings also suggest that, at the very least, membrane ERs share important structural features with the classical ERα.
Figure 4.
FACS analysis with membrane-impermeant E2 and anti-ER antibody. (A) HUVEC were harvested by nonenzymatic cell dissociation and single-cell suspensions of nonpermeabilized cells stained with E2coBSA-FITC and anti-ERα mAb 1D5 plus GAM-PE in the presence of 5% BSA. Background gating of cells stained with FITC and GAM-PE alone was performed as a negative control (99% of cells within left lower quadrant, data not shown). Five thousand cells were analyzed per sample. FL1-Height: FITC fluorescence relative to gated background; FL2-Height: PE fluorescence relative to gated background. Statistical analysis of quadrants is shown. (B) EA.hy926 monolayer cells were harvested and single-cell suspensions of nonpermeabilized cells stained with E2coBSA-FITC in the presence of 5% BSA. Indicated samples were treated on ice with ICI 182,780 or a 20-fold excess of E2 for 1 h before E2coBSA-FITC staining. Five thousand cytometer-acquired events were analyzed per sample.
Although the minority subset distribution of membrane ERs in HUVEC is of substantial interest, this can confound the functional analyses because, as described above, as little as 1% of HUVEC within a given population may be membrane ER positive. Therefore, attempts were made to find established clonal EC lines which are uniformly membrane ER positive. In functional screens, the immortalized EA.hy926 EC line was the most consistent and robust NO producer in response to E2 (data not shown). Fig. 4B demonstrates that EA.hy926 cells are uniformly positive when stained on ice with E2coBSA-FITC in the presence of excess BSA. This staining (binding) was completely abrogated in the presence of the ER antagonist ICI 182,780 or excess E2, supporting the relevance of this membrane-impermeant estrogen binding and ER engagement by E2.
Distinct Immunoreactive ERs Correlate with Functional Responses.
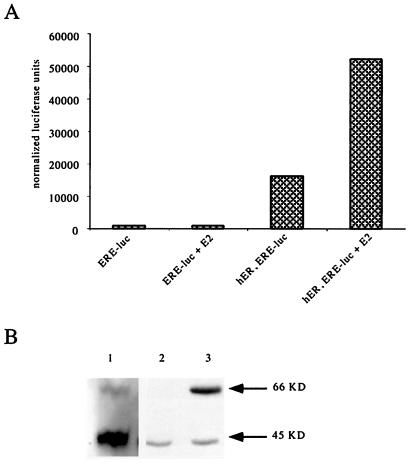
The EA.hy926 cell line is readily transfectable and, for this reason, had recently been used in some attempted ER-mediated transcriptional regulation experiments. Despite their robust response in rapid activation (NO release) assays, the expected genomic responses to E2 were not observed. Fig. 5A demonstrates that a highly E2-sensitive ERE-luciferase reporter construct was uninducible when transfected into EA.hy926 cells. When cotransfecting a full-length ERα expression construct, a strong E2-stimulated transcriptional induction of the ERE was observed (in addition to a small amount of ligand-independent ERE-luciferase activation). This suggests that distinct receptors mediate the rapid, nongenomic and genomic responses to E2 in EC.
Figure 5.
Structure-function correlation between different forms of estrogen receptors. (A) EA.hy926 cells were transiently cotransfected with ERE-luciferase (ERE-luc) and cytomegalovirus β-galactosidase reporter constructs, with or without a full-length ERα expression construct (hER). Twenty-four hours posttransfection and 48 h postestrogen deprivation, transfected monolayers were stimulated with E2 or vehicle control, and luciferase activity was measured in cell extracts after 24 h. Counts represent β-galactosidase-normalized luciferase units. (B) Western blot analysis for ERα (immunoblotting with anti-C-terminal ERα mAb F-10) was performed on total cell lysates obtained from HUVEC (lane 1), EA.hy926 cells (lane 2), and EA.hy926 cells transiently transfected with hER (lane 3). Lane 2 cells are representative of those used in columns 1 and 2 experimental samples (A, above) and lane 3 cells are representative of those used in columns 3 and 4 (A, above).
To evaluate the biochemical correlate of this signaling hierarchy, Western blots were performed on total cellular extracts from HUVEC and EA.hy926 using the F10 anti-ERα C-terminal antibody. Extracts from HUVEC, in which both genomic (1) and rapid signaling responses (21) can be reproducibly triggered by E2, contain two prominent immunoreactive bands: one of the expected 66-kDa mass and the other at 45 kDa (Fig. 5B, lane 1). In contrast, extracts from EA.hy926 cells, in which only rapid signaling occurs, contained only the lower molecular mass 45-kDa molecule (lane 2). Upon EA.hy926 transfection with the ERα expression construct, the expected 66-kDa band was then identified, in addition to the 45-kDa band, and transcriptional activation responses were restored. These findings begin to provide a correlation between distinct endothelial ER structures and their preferential function.
Discussion
The clinical use of estrogen agonists and antagonists has significantly changed the course of hormone-dependent diseases such as breast cancer and postmenopausal osteoporosis. However, the side effects of the agents currently in use are also significant. For example, use of the estrogen-receptor “antagonist” tamoxifen results in rapid progression of osteoporosis. The recent discovery of a number of synthetic estrogens with more desirable tissue-specific agonist/antagonist profiles has led to renewed enthusiasm for hormonal regulation of such diseases. Predictions regarding the effects of any given synthetic estrogen on the multiple systems affected by estrogens (e.g., breast, bone, gonadal, brain, and cardiovascular tissues) will require a better understanding of the pathways through which the multiple cellular responses to estrogens are mediated. For example, breast cancer cell growth has been proposed to be affected both by genomic and nongenomic signaling pathways activated by estradiol (22). The idea that the nongenomic signaling pathway is activated by a cell surface ER may be of critical importance when considering methods of selective drug delivery which may allow us to significantly refine the effects of hormone therapy.
Our data show that stimulation of human vascular EC with both free and membrane-impermeant forms of E2 leads to a rapid, transient, posttranslational activation of MAPK. MAPK activation by estrogen in ovine cells has been reported to occur within 5 min of stimulation (15). We have also observed MAPK activation within 5 min in some experiments (data not shown). However, we have chosen 15-min stimulations for most of our single time point experiments because of better response reproducibility. Some of these differences may be related to delivery of the estrogen to the cell surface. We took great care to ensure that reagent delivery to cells occurred without significant disturbance of the endothelial monolayer, to avoid inadvertent potential activation of MAPKs by mechanical forces. Mechanical stimulation, specifically fluid shear stress, can trigger rapid MAPK activation in bovine aortic EC (23). In this regard, control cells were treated identically, by addition of media with drug carrier alone, to ensure that all cells were subjected to the same low levels of mechanical disturbance at the time of drug delivery.
Steroid-BSA (or other large molecule) conjugates have been used extensively to demonstrate specific plasma membrane binding sites for a variety of steroid hormones (16). Using E2-BSA-FITC, a relatively small percentage (1–30%) of HUVEC was consistently positive in FACS analyses. Routine factor VIII staining demonstrates a much higher degree of EC purity (>98%) in HUVEC cultures used in these experiments. We have previously described a high degree of genetic variability among HUVEC populations, with regard to cytokine responsiveness and adhesion molecule expression (24). The wide range of E2-BSA-FITC positivity could be another manifestation of this genetic heterogeneity. Furthermore, EC derived from different vascular beds can be phenotypically distinct (25). It is possible that specific subsets of cells (all endothelial) within HUVEC cultures are responsible for this minority population of positive cells. However, recent transfection studies in Chinese hamster ovary cells have demonstrated that a single transcript (either ERα or β) can encode for both nuclear and membrane receptors of moderately high affinity and that rapid signaling responses, presumably secondary to engagement of the membrane receptor, can be triggered (5). Either ERα or β expression constructs led to a much greater level of nuclear than membrane expression (30–50:1). Therefore, it is possible that there exists a dynamic pool of ERs that have the ability to translocate to the plasma membrane, although to low levels. Also, although a useful quantitative technique, FACS analysis may be inadequately sensitive to detect low levels of membrane receptors (e.g., tumor necrosis factor and IL-1 receptors) even if these receptors are expressed at levels sufficient to mediate potent signaling responses. Finally, we have performed FACS-based cell sorts to isolate distinct populations of E2-BSA-FITC-positive and -negative HUVEC. These subsets do not maintain their surface ER phenotype, when serially passaged in culture (data not shown), suggesting that the E2-BSA-FITC-positive cells do not represent a fixed subset, but rather a differentiated expression state of a dynamic culture of cells. This is also supported by the uniformity of membrane ER reactivity in the clonal, permanently established EA.hy926 cell line. Ongoing experiments in our laboratory are directed at defining cellular and molecular requirements for this surface expression.
Despite the previously mentioned single transcript data in heterologous experimental systems, it remains unclear at this time whether the membrane E2 binding sites are structurally identical to traditional nuclear ERs. The fact that E2-BSA-FITC-binding HUVEC also bear a membrane epitope (based on mAb recognition in FACS assays) contained within the N terminus of ERα, and that an endothelial protein of a molecular mass distinct from that of classical ERs bears an epitope contained within the C terminus of ERα (based on mAb recognition in Western blots), supports that there exists common structural elements, at least in key molecular domains. Our data correlating the presence of a ≈45-kDa anti-ERα immunoreactive molecule with rapid, E2-stimulated signaling but not transcriptional responses support that this “ER form” is functionally significant. This lower molecular mass is consistent with various ERα splice variants previously described (26). However, the standard receptors do not conform to a transmembrane molecular structure. Hydrophobicity analysis of ERα (and β) suggests that the only portion of the molecule capable of membrane insertion is the ligand binding domain, which is too short to form a hairpin bend. This is in conflict with the prior findings of extracellular reactivity to both anti-N- and -C-terminal antibodies (19) and to our antibody reactivity data. Thus, it is possible that the surface ER is composed of a multimeric complex, including at least part of a traditional ER which is largely extracellular, but bound to an as yet unidentified transmembrane protein and coupled to signaling molecules. Alternatively, the surface ER may have several common structural features with, but may be distinct from, the described intracellular ERs.
No system better exemplifies the wide spectrum of the effects of estrogen than the cardiovascular system. The gender-based differences in cardiovascular disease rates are clearly hormonally mediated. Yet, our understanding of many of the estrogen effects on relevant tissues, especially those effects which occur rapidly, is underdeveloped. Our current data provide evidence that rapid human vascular endothelial cell responses to estrogen can be mediated by estrogen binding to a cell surface form of ER. Further definition of this receptor and its signal transduction mechanism will lead to novel prevention and treatment approaches to cardiovascular disease.
Acknowledgments
We gratefully acknowledge Lynn O'Donnell, Louise Benson, and Gwen Davis for assistance with cell culture and Dana Brenckle for assistance with manuscript preparation. This work was supported by National Institutes of Health Grant RO1HL61782 and an educational grant from Parke-Davis.
Abbreviations
- EC
endothelial cell
- E2
17β-estradiol
- E2coBSA
β-estradiol-6-(O-carboxymethyl)oxime BSA
- E2hsBSA
β-estradiol 17-hemisuccinate BSA
- ER
estrogen receptor
- MAPK
mitogen-activated protein kinase
- HUVEC
human umbilical vein endothelial cells
- GAM
goat anti-mouse
- PE
phycoerythrin
References
- 1.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa W C, Bender J R. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 2.Blackmore P F, Neulen J, Lattanzio F, Beebe S J. J Biol Chem. 1991;266:18655–18659. [PubMed] [Google Scholar]
- 3.Szego C M, Pietras R J. Nature (London) 1985;317:88–89. doi: 10.1038/317088a0. [DOI] [PubMed] [Google Scholar]
- 4.Pietras R J, Szego C M. Nature (London) 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 5.Razandi M, Pedram A, Greene G L, Levin E R. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 6.Pfau S, Leitenberg D, Rinder H, Smith B R, Pardi R, Bender J R. J Cell Biol. 1995;128:969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgell C J, McDonald C C, Graham J B. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papapetropoulos A, Marczin N, Snead M D, Cheng C, Milici A, Catravas J D. Hypertension. 1994;23:476–484. doi: 10.1161/01.hyp.23.4.476. [DOI] [PubMed] [Google Scholar]
- 9.Klinge C M, Silver B F, Driscoll M D, Sathya G, Bambara R A, Hilf R. J Biol Chem. 1997;272:31465–31474. doi: 10.1074/jbc.272.50.31465. [DOI] [PubMed] [Google Scholar]
- 10.Theulaz I, Hipskind R, ten Heggeler-Bordier B, Green S, Kumar V, Chambon P, Wahli W. EMBO J. 1988;7:1653–1660. doi: 10.1002/j.1460-2075.1988.tb02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell K S, Haynes M P, Caulin-Glaser T, Rosneck J, Sessa W C, Bender J. J Biol Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 13.Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H. Biochem Biophys Res Commun. 1997;235:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- 14.Watters J J, Campbell J S, Cunningham M J, Krebs E G, Dorsa D M. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Yuhanna I S, Galcheva-Gargova Z, Karas R H, Mendelsohn M E, Shaul P W. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Ali A, Ramirez V D. J Psychiatry Neurosci. 1996;21:187–197. [PMC free article] [PubMed] [Google Scholar]
- 17.Dembinski T C, Leung C K, Shiu R P. Cancer Res. 1985;45:3083–3089. [PubMed] [Google Scholar]
- 18.Watson C S, Pappas T C, Gametchu B. Environ Health Perspect. 1995;103, Suppl 7:41–50. doi: 10.1289/ehp.95103s741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappas T C, Gametchu B, Watson C S. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 20.Germain P S, Metezeau P, Tiefenauer L X, Kiefer H, Ratinaud M H, Habrioux G. Anticancer Res. 1993;13:2347–2353. [PubMed] [Google Scholar]
- 21.Caulin-Glaser T, Watson C A, Pardi R, Bender J R. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliaccio A, Pagano M, Auricchio F. Oncogene. 1993;8:2183–2191. [PubMed] [Google Scholar]
- 23.Tseng H, Peterson T E, Berk B C. Circ Res. 1995;77:869–878. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 24.Bender J R, Sadeghi M M, Watson C, Pfau S, Pardi R. Proc Natl Acad Sci USA. 1994;91:3994–3998. doi: 10.1073/pnas.91.9.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, West D C, Ager A. Differentiation. 1987;36:57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Jazaeri O, Shupnik M A, Jazaeri A A, Rice L W. Gynecol Oncol. 1999;74:38–47. doi: 10.1006/gyno.1999.5404. [DOI] [PubMed] [Google Scholar]