Abstract
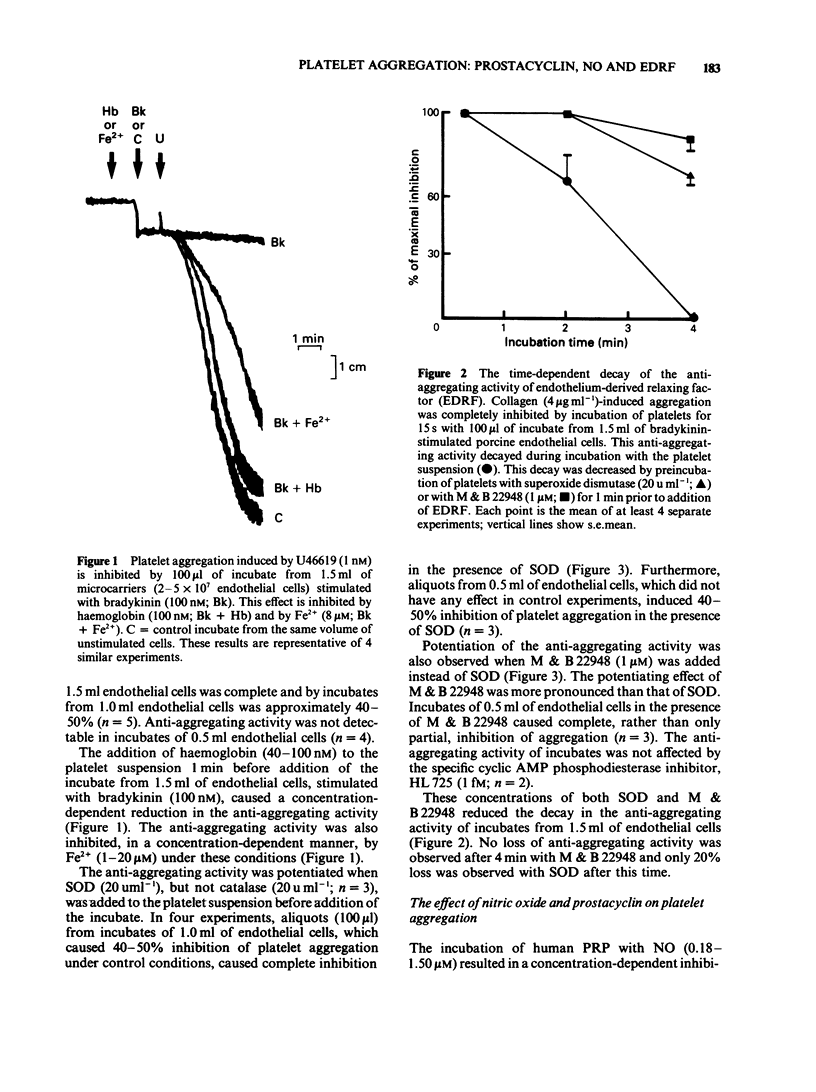
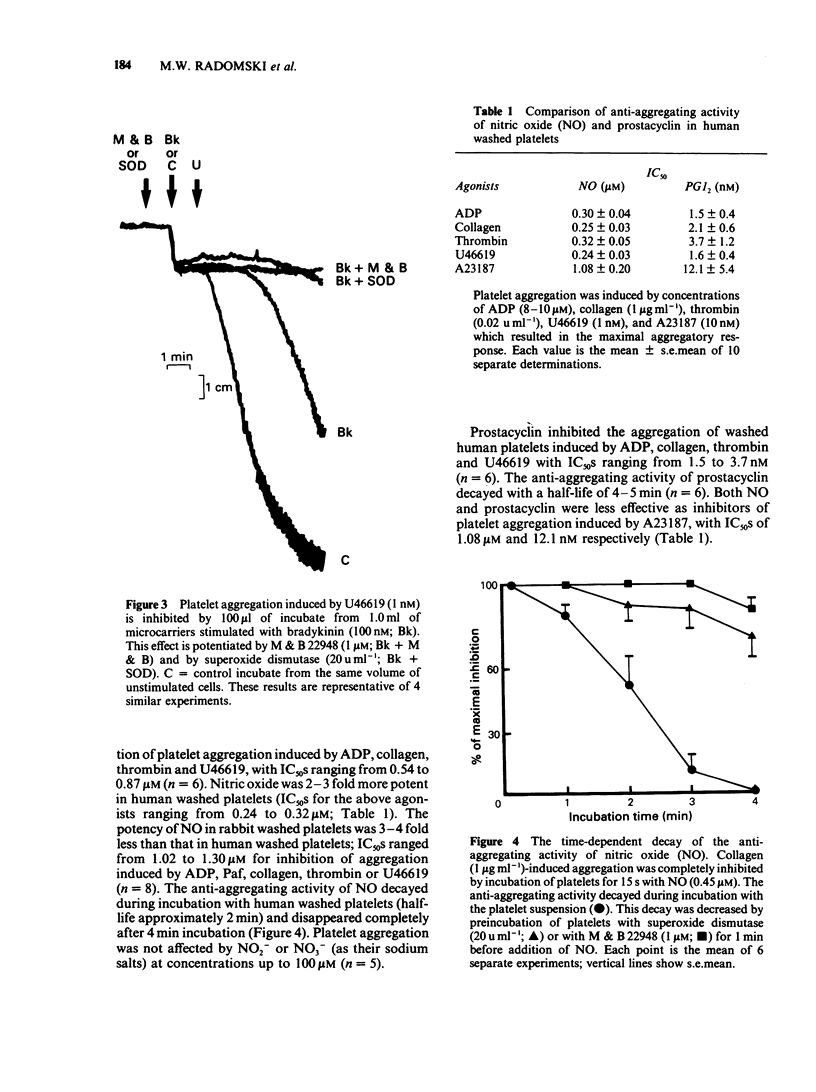
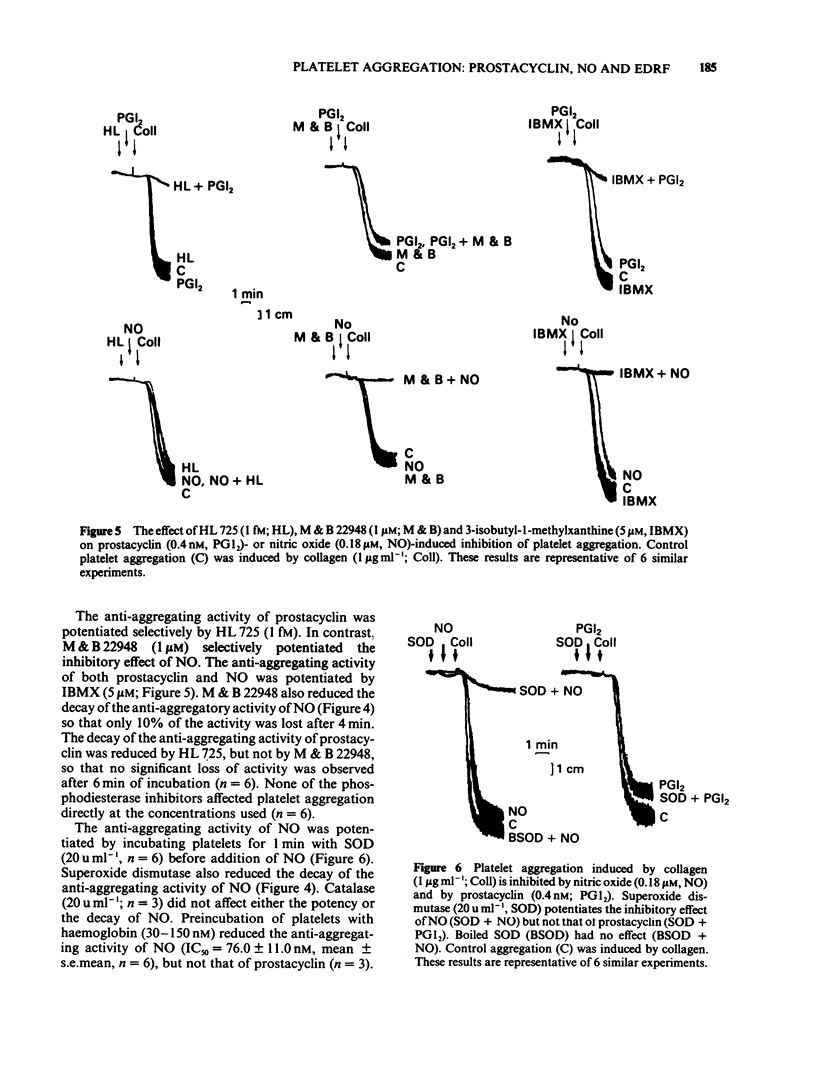
1 The pharmacological effects of endothelium-derived relaxing factor (EDRF), nitric oxide (NO) and prostacyclin on human and rabbit platelets were examined. 2 EDRF is released from porcine aortic endothelial cells, cultured on microcarriers and treated with indomethacin, in sufficient quantities to inhibit platelet aggregation induced by 9,11-dideoxy-9 alpha, 11 alpha-methano epoxy-prostaglandin F2 alpha (U46619) and collagen. 3 The anti-aggregating activity of EDRF was potentiated by M&B 22948, a selective inhibitor of cyclic GMP phosphodiesterase, and by superoxide dismutase (SOD) and was inhibited by haemoglobin and Fe2+. 4 Both NO and prostacyclin inhibited platelet aggregation. 5 The anti-aggregatory activity of NO, but not that of prostacyclin, was potentiated by M&B 22948 and by SOD and was inhibited by haemoglobin and Fe2+. Thus NO is a potent inhibitor of platelet aggregation whose activity on platelets mimics that of EDRF. 6 It is likely that the inhibitory effect of NO on platelets represents the action of endogenous EDRF and therefore this substance, together with prostacyclin, is a regulator of platelet-vessel wall interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons R. M., Lee M. R., Bliss E. L., Asbury A. C., Cook D., Brown V. Failure of superoxide dismutase to alter equine arachidonic acid-induced platelet aggregation, in vitro or ex vivo. Am J Vet Res. 1985 May;46(5):1104–1106. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furlong B., Henderson A. H., Lewis M. J., Smith J. A. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987 Apr;90(4):687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Silk S. T., Safier L. B., Ullman H. L. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977 Jan;59(1):149–158. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Myers C. B., Ohlstein E. H., Ballot B. A., Hyman A. L., Kadowitz P. J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983 May;23(3):653–664. [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Ruppert D., Weithmann K. U. HL 725, an extremely potent inhibitor of platelet phosphodiesterase and induced platelet aggregation in vitro. Life Sci. 1982 Nov 8;31(19):2037–2043. doi: 10.1016/0024-3205(82)90095-9. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Schafer A. I., Alexander R. W., Handin R. I. Inhibition of platelet function by organic nitrate vasodilators. Blood. 1980 Apr;55(4):649–654. [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Vargas J. R., Radomski M., Moncada S. The use of prostacyclin in the separation from plasma and washing of human platelets. Prostaglandins. 1982 Jun;23(6):929–945. doi: 10.1016/0090-6980(82)90135-6. [DOI] [PubMed] [Google Scholar]


