Abstract
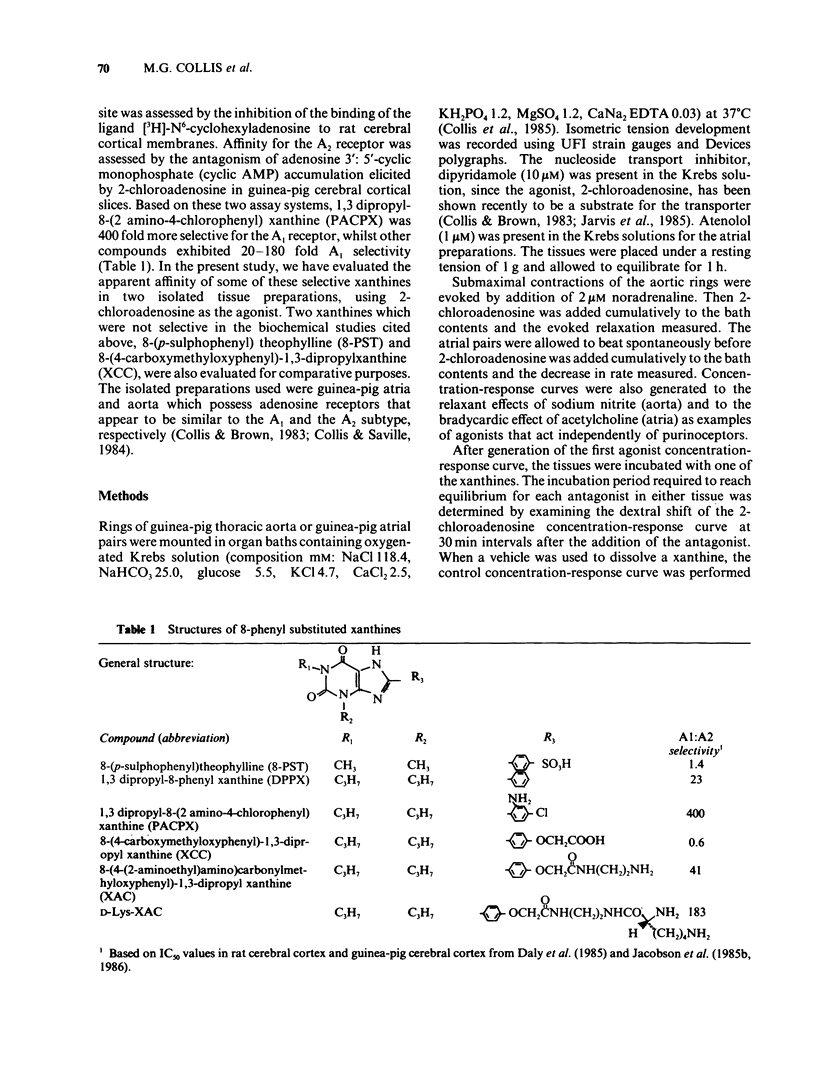
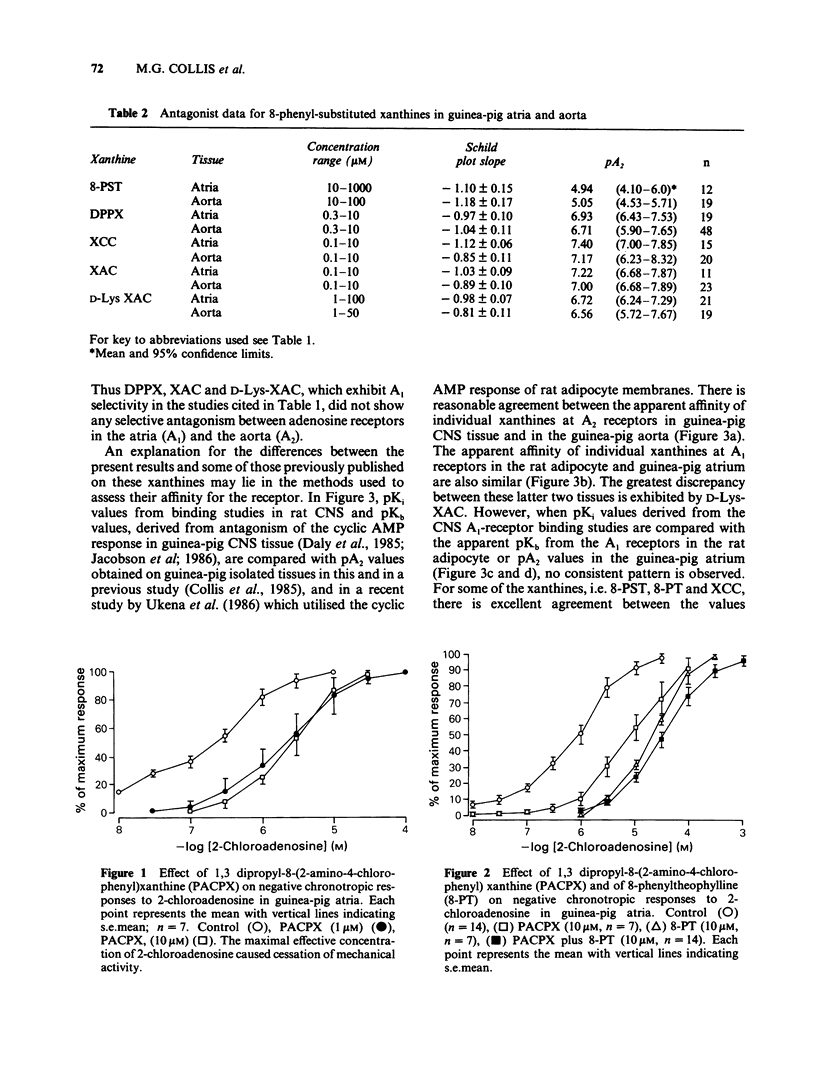
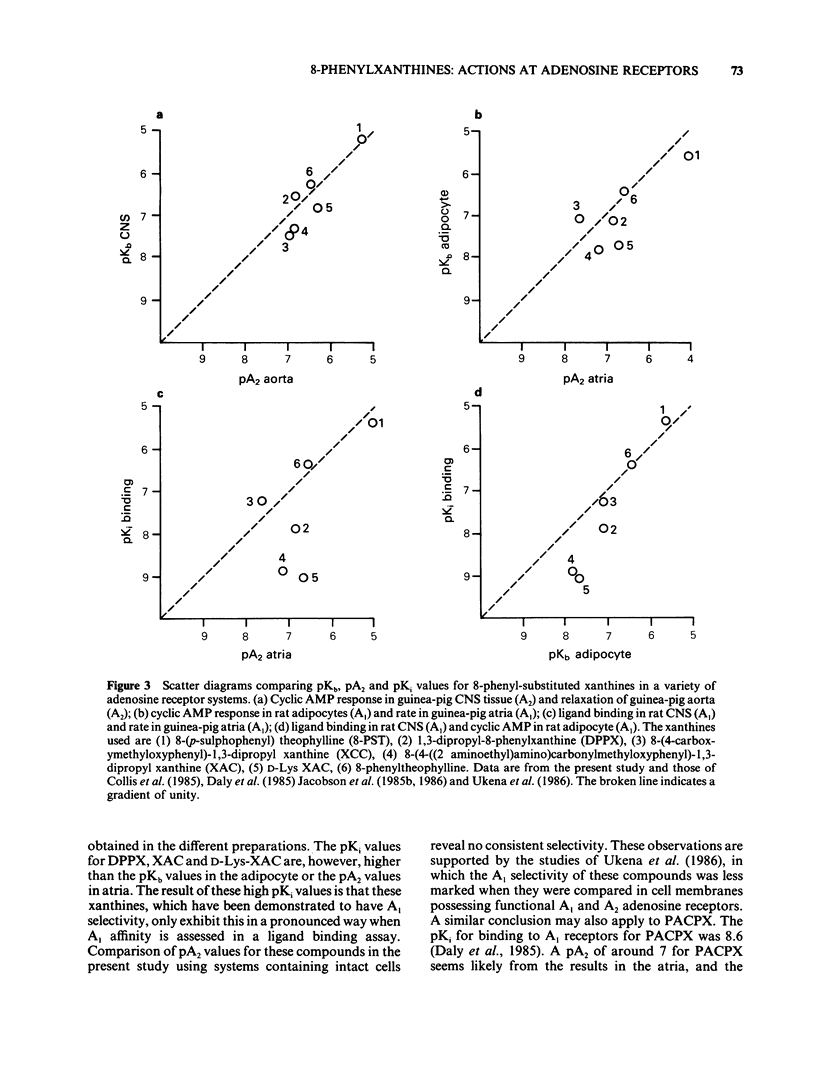
1 Some 8-phenyl-substituted, 1,3 dipropyl xanthines have previously been demonstrated to have a 20-400 fold greater affinity for A1 binding sites in rat CNS membranes than for A2 adenosine receptors in intact CNS cells from guinea-pigs. In the present study these compounds (1,3, dipropyl-8-phenylxanthine: DPPX; 1,3 dipropyl-8-(2 amino-4-chlorophenyl) xanthine: PACPX; 8-(4-(2-amino-ethyl)amino) carbonyl methyl oxyphenyl)-1,3-dipropylxanthine: XAC; and D-Lys-XAC) together with two that have not been reported to exhibit A1-receptor selectively (8-(p-sulphophenyl)theophylline: 8-PST; 8-(4-carboxy methyl oxyphenyl)-1,3-dipropylxanthine: XCC) have been evaluated as antagonists of the effects of 2-chloroadenosine in two isolated cardiovascular tissues. 2 The isolated tissues used were guinea-pig atria (bradycardic response) and aorta (relaxation), which are thought to possess A1 and A2 adenosine receptors, respectively. 3 All the xanthines antagonized responses evoked by 2-chloroadenosine in both tissues but did not affect responses evoked by acetylcholine (atria) or sodium nitrite (aorta). 4 The xanthines, 8-PST, XAC, D-Lys XAC, XCC and DPPX appeared to be competitive antagonists of the effects of 2-chloroadenosine, as Schild plot slopes did not differ significantly from unity. The 1,3-dipropyl substituted compounds had pA2 values from 6.5 to 7.4 and were more potent than the 1,3 dimethyl substituted 8-PST (pA2 4.9 to 5). 5 For individual xanthines, there was no difference between pA2 values obtained in the atria and in the aorta.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Hoyle C. H. PACPX--a substituted xanthine--antagonizes both the A1 and A2 subclasses of the P1-purinoceptor: antagonism of the A2 subclass is competitive but antagonism of the A1 subclass is not. Br J Pharmacol. 1985 May;85(1):291–296. doi: 10.1111/j.1476-5381.1985.tb08859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G., Brown C. M. Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol. 1983 Dec 9;96(1-2):61–69. doi: 10.1016/0014-2999(83)90529-0. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Palmer D. B., Saville V. L. Comparison of the potency of 8-phenyltheophylline as an antagonist at A1 and A2 adenosine receptors in atria and aorta from the guinea-pig. J Pharm Pharmacol. 1985 Apr;37(4):278–280. doi: 10.1111/j.2042-7158.1985.tb05063.x. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Shamim M. T., Butts-Lamb P., Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985 Apr;28(4):487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Thompson R. D., Kusachi S., Bugni W. J., Olsson R. A. Structure-activity relationships for N6-substituted adenosines at a brain A1-adenosine receptor with a comparison to an A2-adenosine receptor regulating coronary blood flow. Biochem Pharmacol. 1986 Aug 1;35(15):2467–2481. doi: 10.1016/0006-2952(86)90042-0. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. A functionalized congener approach to adenosine receptor antagonists: amino acid conjugates of 1,3-dipropylxanthine. Mol Pharmacol. 1986 Feb;29(2):126–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985 Sep;28(9):1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson K. A., Kirk K. L., Daly J. W., Jonzon B., Li Y. O., Fredholm B. B. A novel 8-phenyl-substituted xanthine derivative is a selective antagonist at adenosine A1-receptors in vivo. Acta Physiol Scand. 1985 Oct;125(2):341–342. doi: 10.1111/j.1748-1716.1985.tb07725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Martin B. W., Ng A. S. 2-Chloroadenosine, a permeant for the nucleoside transporter. Biochem Pharmacol. 1985 Sep 15;34(18):3237–3241. doi: 10.1016/0006-2952(85)90340-5. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P. The relative contribution of affinity and efficacy to agonist activity: organ selectivity of noradrenaline and oxymetazoline with reference to the classification of drug receptors. Br J Pharmacol. 1984 Jan;81(1):131–141. doi: 10.1111/j.1476-5381.1984.tb10753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Ukena D., Daly J. W., Kirk K. L., Jacobson K. A. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986 Mar 3;38(9):797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D., Jacobson K. A., Padgett W. L., Ayala C., Shamim M. T., Kirk K. L., Olsson R. O., Daly J. W. Species differences in structure-activity relationships of adenosine agonists and xanthine antagonists at brain A1 adenosine receptors. FEBS Lett. 1986 Dec 1;209(1):122–128. doi: 10.1016/0014-5793(86)81096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


