Abstract
1 Indomethacin (10(-4)M) causes marked augmentation of O-2 release from human neutrophils when these are stimulated by either 1,oleoyl-2,acetylglycerol or the divalent cation ionophore, A23187, the concentration-response curve for each agent being shifted to the left and the maximum response to each increased. 2 The diacylglycerol kinase inhibitor, R59022 (10(-5)M) has effects very similar to those of indomethacin on both the 1,oleoyl-2,acetylglycerol-induced and the A23187-induced concentration-response curves for O-2 generation. 3 The diacylglycerol lipase inhibitor, RHC80267 (10(-5 M) on the other hand, has a similar effect to indomethacin on 1,oleoyl-2,acetylglycerol-induced O2- generation but, unlike indomethacin, has no effect on A23187-induced O2- generation. Comparison of the effects of these three agents provides a clue to the locus of the action of indomethacin in increasing superoxide release, suggesting that it may act as a diacylglycerol kinase inhibitor. A component of diacylglycerol lipase inhibition may also be present. It is suggested that these results could have relevance for the use of indomethacin as an anti-inflammatory agent in chronic rheumatoid diseases.
Full text
PDF

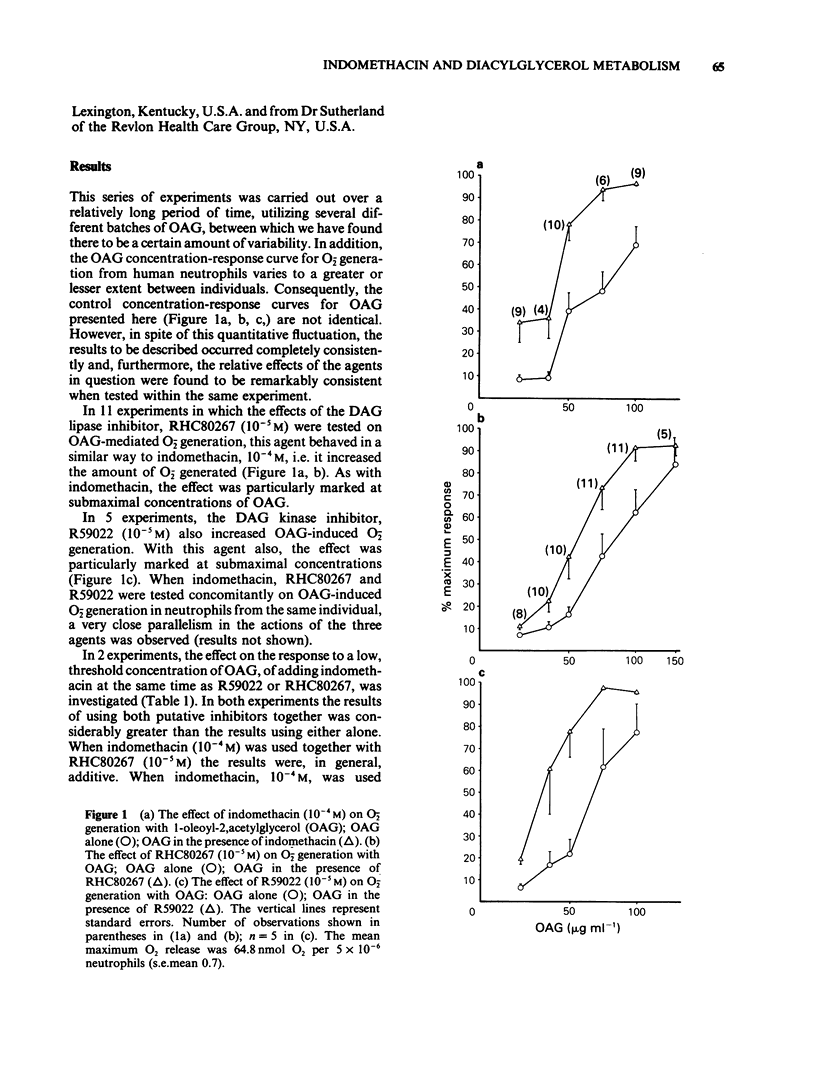
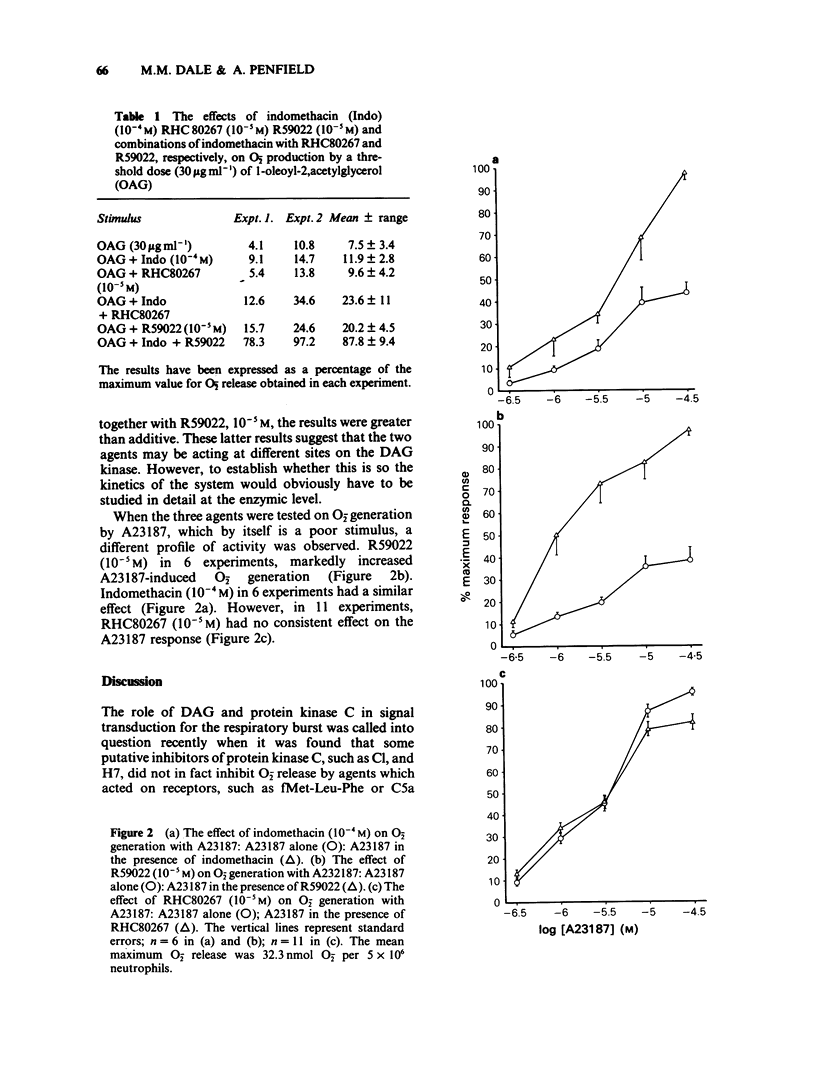


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Unsaturated fatty acids as second messengers of superoxide generation by macrophages. Cell Immunol. 1983 Jul 15;79(2):240–252. doi: 10.1016/0008-8749(83)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. Stimulus-secretion coupling in rabbit neutrophils is not mediated by phosphatidylinositol breakdown. Nature. 1980 Nov 20;288(5788):275–277. doi: 10.1038/288275a0. [DOI] [PubMed] [Google Scholar]
- Dale M. M., Penfield A. Superoxide generation by either 1-oleoyl-2-acetylglycerol or A23187 in human neutrophils is enhanced by indomethacin. FEBS Lett. 1985 Jun 3;185(1):213–217. doi: 10.1016/0014-5793(85)80772-9. [DOI] [PubMed] [Google Scholar]
- Dale M. M., Penfield A. Synergism between phorbol ester and A23187 in superoxide production by neutrophils. FEBS Lett. 1984 Sep 17;175(1):170–172. doi: 10.1016/0014-5793(84)80592-x. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Marasco W. A., Elgas L. J., Ward P. A. Stimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion production. Am J Pathol. 1984 Apr;115(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Gay J. C., English D., Lukens J. N. Stimulation of neutrophil oxidative metabolism by indomethacin. Agents Actions. 1985 Jul;16(5):336–341. doi: 10.1007/BF01982869. [DOI] [PubMed] [Google Scholar]
- Gay J. C., Lukens J. N., English D. K. Differential inhibition of neutrophil superoxide generation by nonsteroidal antiinflammatory drugs. Inflammation. 1984 Jun;8(2):209–222. doi: 10.1007/BF00916096. [DOI] [PubMed] [Google Scholar]
- Gerard C., McPhail L. C., Marfat A., Stimler-Gerard N. P., Bass D. A., McCall C. E. Role of protein kinases in stimulation of human polymorphonuclear leukocyte oxidative metabolism by various agonists. Differential effects of a novel protein kinase inhibitor. J Clin Invest. 1986 Jan;77(1):61–65. doi: 10.1172/JCI112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muid R. E., Penfield A., Dale M. M. The diacylglycerol kinase inhibitor, R59022, enhances the superoxide generation from human neutrophils induced by stimulation of fMet-Leu-Phe, IgG and C3b receptors. Biochem Biophys Res Commun. 1987 Mar 13;143(2):630–637. doi: 10.1016/0006-291x(87)91400-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Penfield A., Dale M. M. Synergism between A23187 and 1-oleoyl-2-acetyl-glycerol in superoxide production by human neutrophils. Biochem Biophys Res Commun. 1984 Nov 30;125(1):332–336. doi: 10.1016/s0006-291x(84)80372-1. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Indomethacin-induced accumulation of diglyceride in activated human platelets. The role of diglyceride lipase. J Biol Chem. 1980 Mar 25;255(6):2259–2262. [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Superoxide release by neutrophils: synergistic effects of a phorbol ester and a calcium ionophore. Biochem Biophys Res Commun. 1984 Jul 31;122(2):734–739. doi: 10.1016/s0006-291x(84)80095-9. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wright C. D., Hoffman M. D. The protein kinase C inhibitors H-7 and H-9 fail to inhibit human neutrophil activation. Biochem Biophys Res Commun. 1986 Mar 28;135(3):749–755. doi: 10.1016/0006-291x(86)90992-7. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]


