Abstract
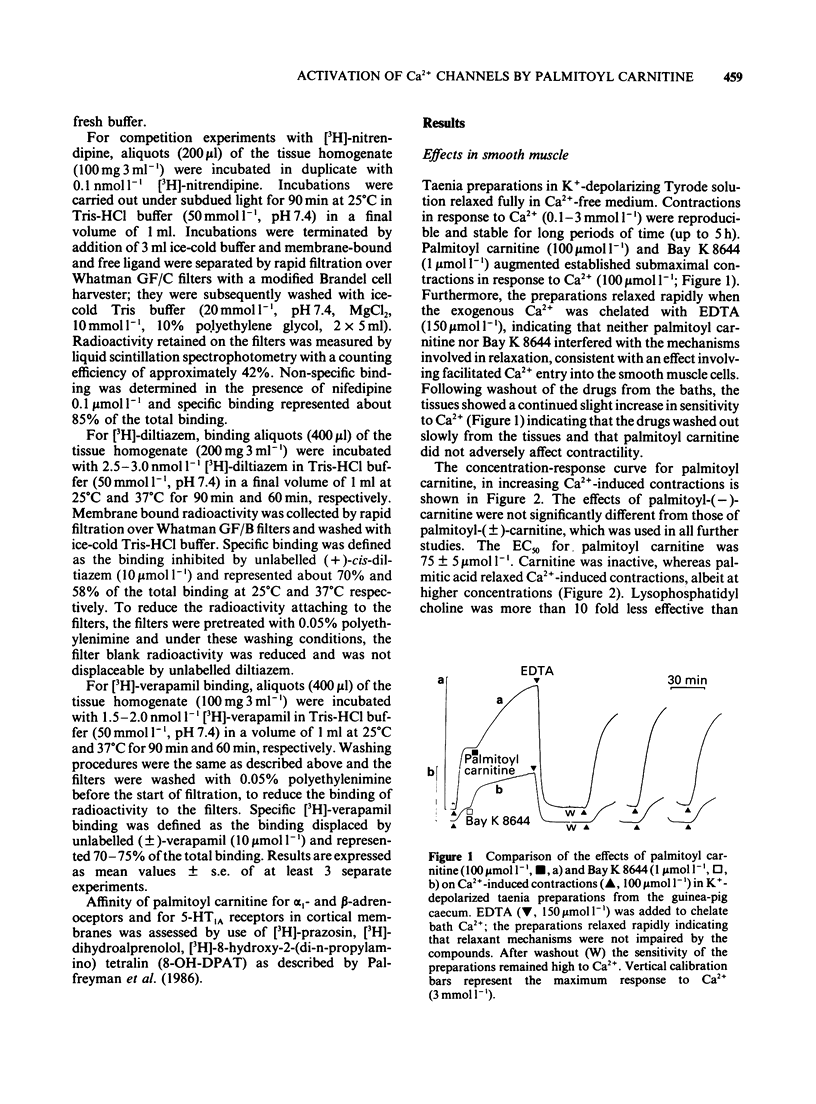
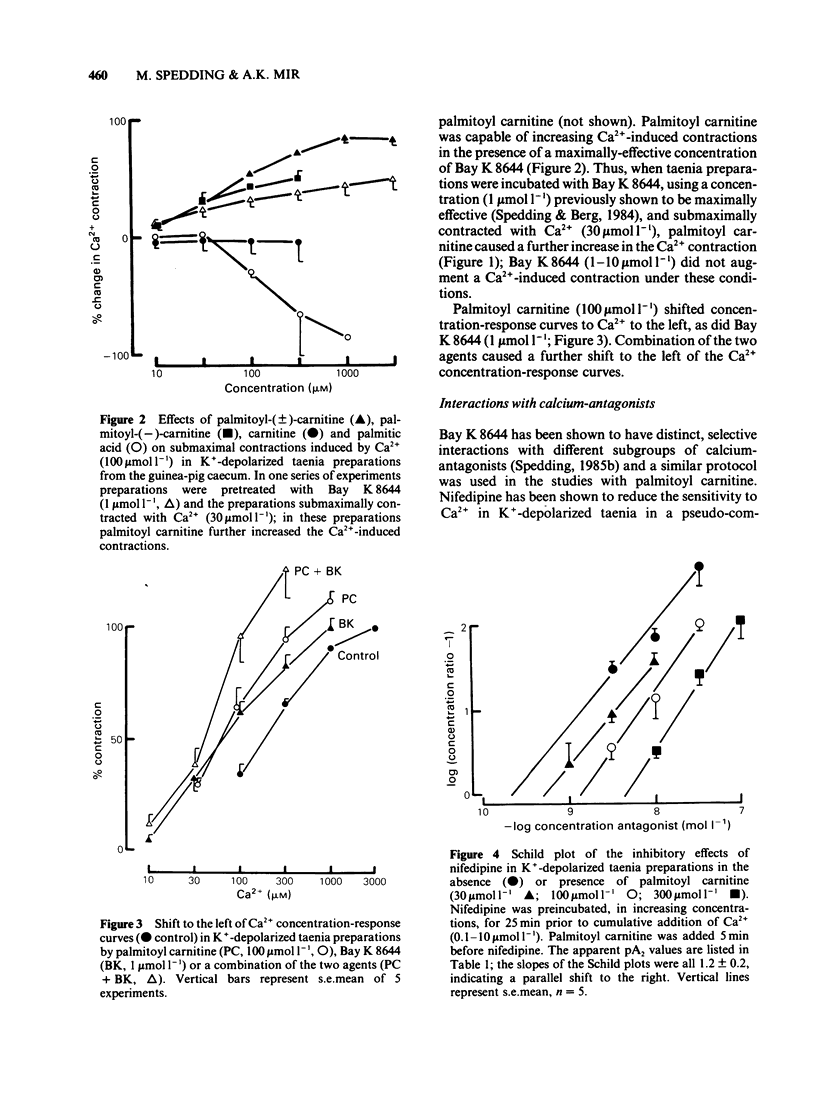
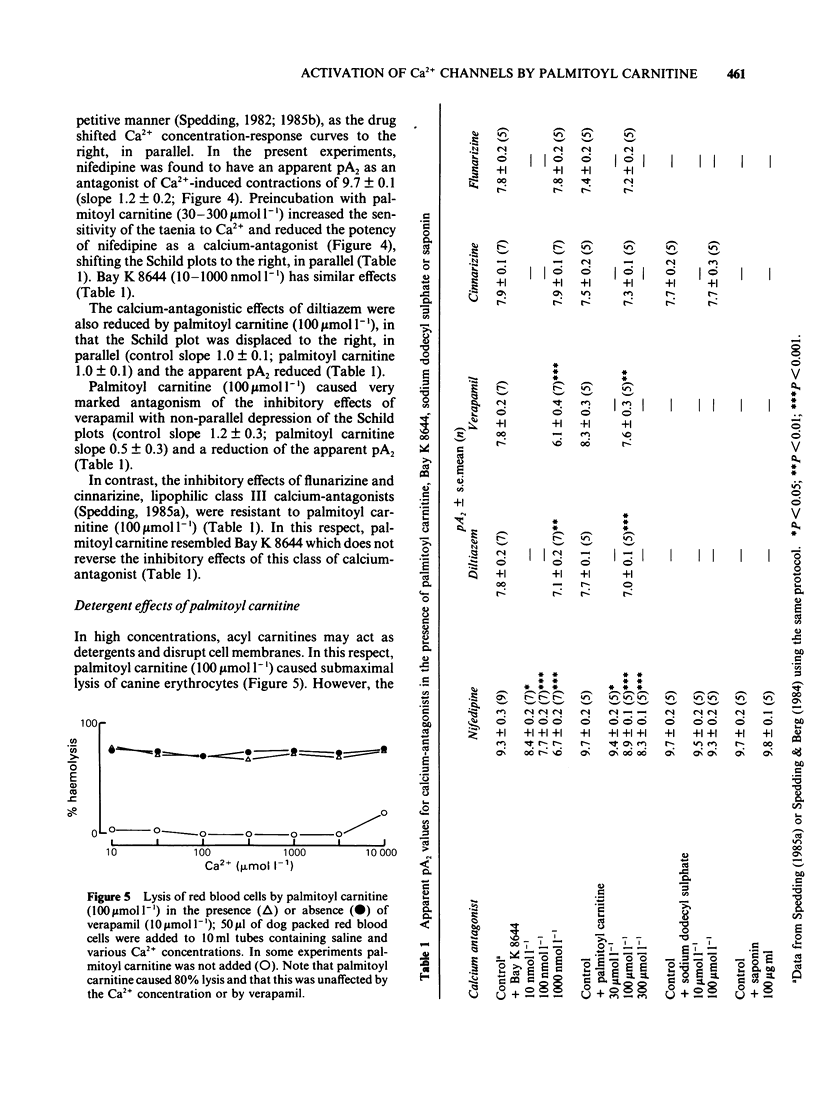
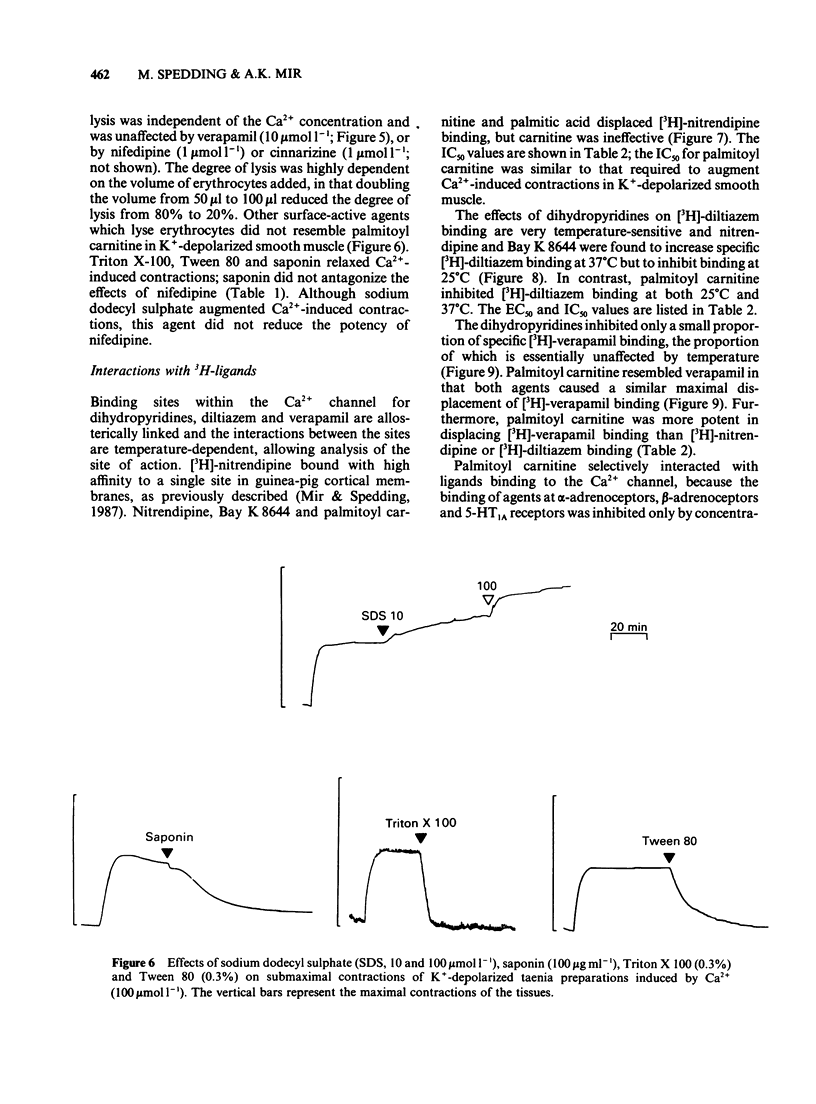
1 Palmitoyl carnitine, a lipid metabolite which accumulates in cytoplasmic membranes during ischaemia, has been shown to resemble the Ca2+ channel activator, Bay K 8644, in K+-depolarized smooth muscle. Palmitoyl carnitine caused concentration-dependent (1-1000 mumol l-1) augmentations in the sensitivity to Ca2+ of K+-depolarized taenia preparations from the guinea-pig caecum. The (+/-)-isomer was equieffective with the (-)-isomer, whereas carnitine was ineffective and palmitic acid relaxed the tissues. The shift to the left of Ca2+ concentration-response curves induced by palmitoyl carnitine (100 mumol l-1) was additive with that of Bay K 8644 (1 mumol l-1). 2 The interactions of palmitoyl carnitine with the different classes of calcium-antagonist were similar to those seen with Bay K 8644. Schild plots of the calcium-antagonist effects of nifedipine were shifted to the right following preincubation of the taenia with palmitoyl carnitine (30-300 mumol l-1). The inhibitory effects of verapamil were especially sensitive to palmitoyl carnitine (100 mumol l-1). Whereas the potency of diltiazem as a calcium-antagonist was reduced by palmitoyl carnitine (100 mumol l-1), the inhibitory effects of the lipophilic class III calcium-antagonists, cinnarizine and flunarizine, were entirely resistant to palmitoyl carnitine (100 mumol l-1). 3 Although palmitoyl carnitine has detergent properties in high concentrations and lyses red blood cells, these effects were not Ca2+-dependent, nor were they modified by calcium-antagonists. Other detergents did not have selective interactions with Ca2+ channels. 4 Palmitoyl carnitine inhibited [3H]-nitrendipine, [3H]-verapamil and [3H]-diltiazem binding to rat cortical membranes with IC50 values (mumol l-1) of 120 +/- 1, 95 +/- 17 and 120 +/- 15 mumol l-1 respectively. The inhibition showed little temperature-dependence, in contrast to that of Bay K 8644, except for a small reduction in the IC50 value for [3H]-verapamil binding at 37 degrees C (42 +/- 5 mumol l-1). Palmitoyl carnitine interacted selectively with the Ca2+ channel, in that effects on ligand binding to alpha-adrenoceptors, beta-adrenoceptors and 5-HT1A receptors occurred only at 5-10 fold higher concentrations. 5 It is concluded that palmitoyl carnitine, at concentrations which have previously been shown to occur in the cytoplasm during myocardial ischaemia, may interact directly with Ca2+ channels and may therefore be considered as an endogenous modulator of channel function. The site of action differs from that of other agents.
Full text
PDF

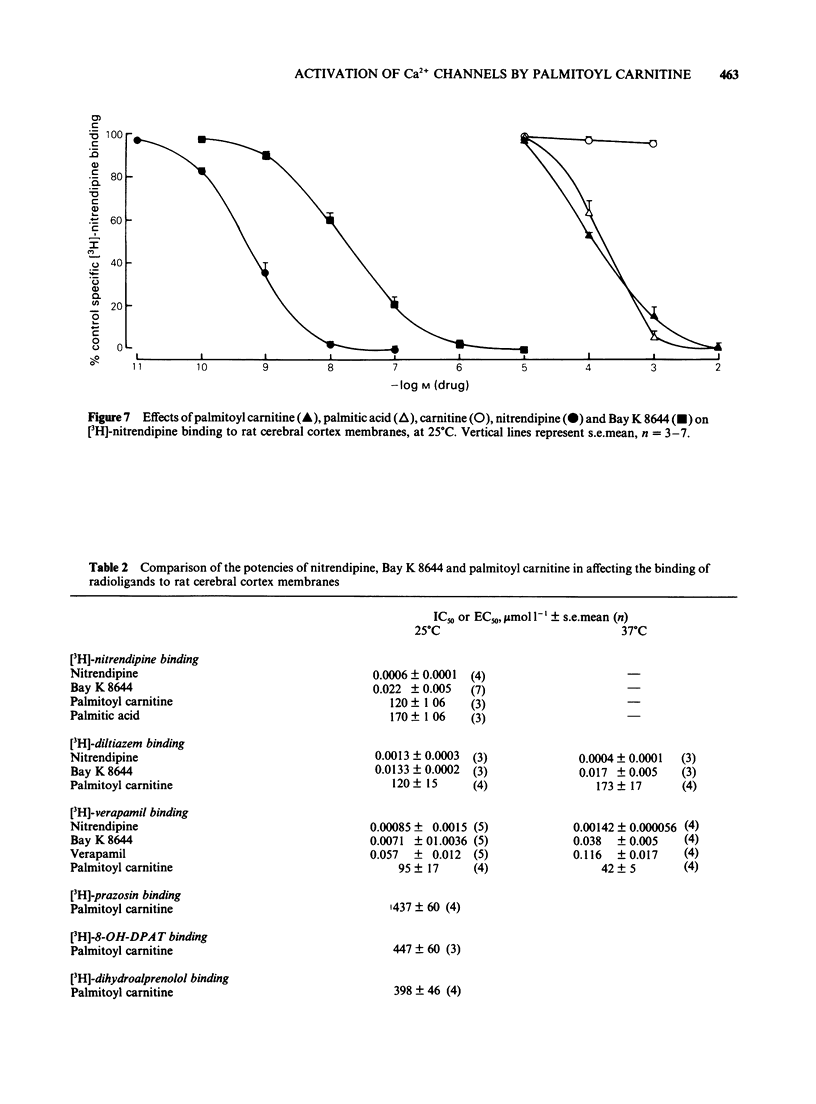
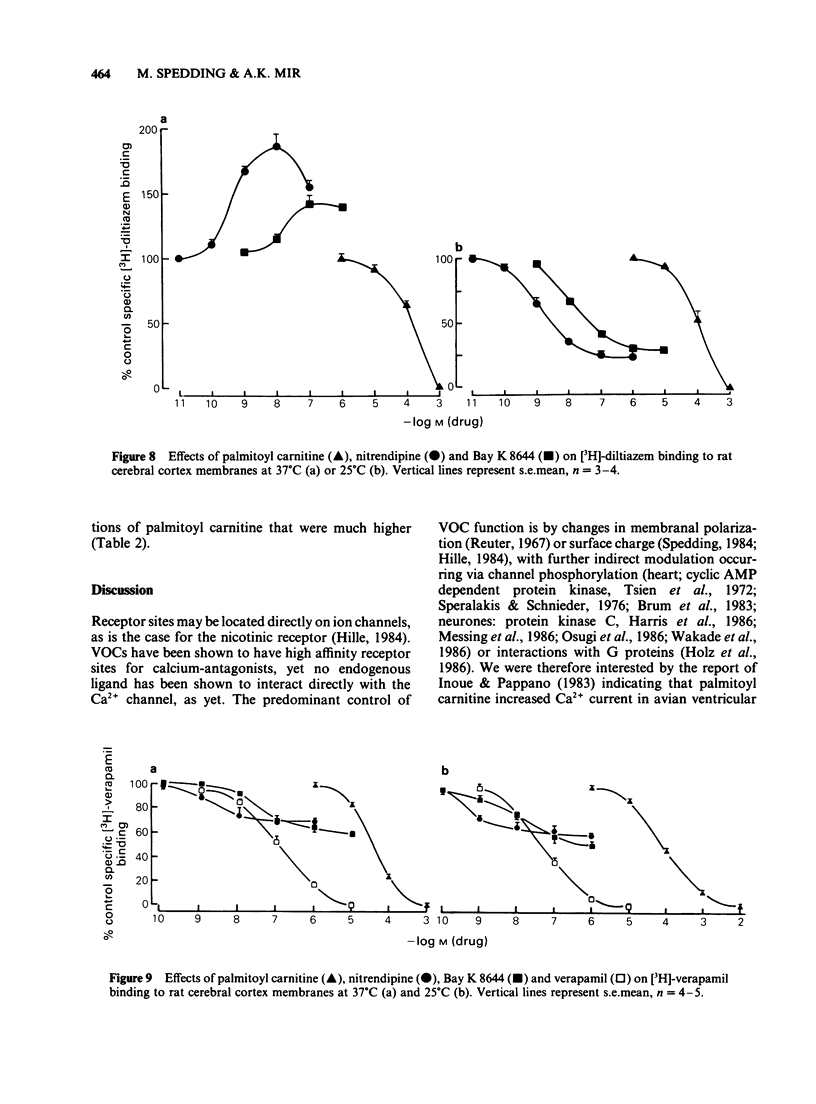




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. J., Cohen D. W., Gupte S., Johnson J. D., Wallick E. T., Wang T., Schwartz A. In vitro effects of palmitylcarnitine on cardiac plasma membrane Na,K-ATPase, and sarcoplasmic reticulum Ca2+-ATPase and Ca2+ transport. J Biol Chem. 1979 Dec 25;254(24):12404–12410. [PubMed] [Google Scholar]
- Brecher P. The interaction of long-chain acyl CoA with membranes. Mol Cell Biochem. 1983;57(1):3–15. doi: 10.1007/BF00223520. [DOI] [PubMed] [Google Scholar]
- Brum G., Flockerzi V., Hofmann F., Osterrieder W., Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983 Jul;398(2):147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Cain M. E., Crafford W. A., Jr, Gross R. W., Sobel B. E. Electrophysiological effects of amphiphiles on canine purkinje fibers. Implications for dysrhythmia secondary to ischemia. Circ Res. 1981 Aug;49(2):354–363. doi: 10.1161/01.res.49.2.354. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Lee B. I., Gross R. W., Keim C. R., Sobel B. E. Pathophysiological concentrations of lysophosphatides and the slow response. Am J Physiol. 1982 Aug;243(2):H187–H195. doi: 10.1152/ajpheart.1982.243.2.H187. [DOI] [PubMed] [Google Scholar]
- Eskelinen S., Mela M. Cation permeability and mechanical properties of the erythrocyte membrane under the influence of lysophosphatidylcholine (LPC) in isotonic and hypotonic media. Acta Physiol Scand. 1984 Dec;122(4):527–534. doi: 10.1111/j.1748-1716.1984.tb07541.x. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. Characterization of the Ca2+ coordination site regulating binding of Ca2+ channel inhibitors d-cis-diltiazem, (+/-)bepridil and (-)desmethoxyverapamil to their receptor site in skeletal muscle transverse tubule membranes. Biochem Biophys Res Commun. 1985 Oct 15;132(1):49–55. doi: 10.1016/0006-291x(85)90986-6. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Goll A., Striessnig J., Zernig G. Calcium channels and calcium channel drugs: recent biochemical and biophysical findings. Arzneimittelforschung. 1985;35(12A):1917–1935. [PubMed] [Google Scholar]
- Grima M., Schwartz J., Spach M. O., Velly J. Anti-anginal arylalkylamines and sodium channels: [3H]-batrachotoxinin-A 20-alpha-benzoate and [3H]-tetracaine binding. Br J Pharmacol. 1986 Dec;89(4):641–646. doi: 10.1111/j.1476-5381.1986.tb11168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Kongsamut S., Miller R. J. Protein kinase C mediated regulation of calcium channels in PC-12 pheochromocytoma cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1298–1305. doi: 10.1016/0006-291x(86)90391-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Barnes K. C., Kinkade J. M., Jr, Vogler W. R., Shoji M., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein phosphorylation system in various types of leukemic cells from human patients and in human leukemic cell lines HL60 and K562, and its inhibition by alkyl-lysophospholipid. Cancer Res. 1983 Jun;43(6):2955–2961. [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Inoue D., Pappano A. J. L-palmitylcarnitine and calcium ions act similarly on excitatory ionic currents in avian ventricular muscle. Circ Res. 1983 Jun;52(6):625–634. doi: 10.1161/01.res.52.6.625. [DOI] [PubMed] [Google Scholar]
- Katoh N., Wrenn R. W., Wise B. C., Shoji M., Kuo J. F. Substrate proteins for calmodulin-sensitive and phospholipid-sensitive Ca2+-dependent protein kinases in heart, and inhibition of their phosphorylation by palmitoylcarnitine. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4813–4817. doi: 10.1073/pnas.78.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Knabb M. T., Saffitz J. E., Corr P. B., Sobel B. E. The dependence of electrophysiological derangements on accumulation of endogenous long-chain acyl carnitine in hypoxic neonatal rat myocytes. Circ Res. 1986 Feb;58(2):230–240. doi: 10.1161/01.res.58.2.230. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Oliver M. F. Serum-free-fatty-acids after acute myocardial infarction and cerebral vascular occlusion. Lancet. 1966 Jul 16;2(7455):122–127. doi: 10.1016/s0140-6736(66)92420-2. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J., Nellis S., Neely J. R. Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ Res. 1978 Oct;43(4):652–661. doi: 10.1161/01.res.43.4.652. [DOI] [PubMed] [Google Scholar]
- Linden J., Brooker G. The influence of resting membrane potential on the effect of verapamil on atria. J Mol Cell Cardiol. 1980 Mar;12(3):325–331. doi: 10.1016/0022-2828(80)90044-9. [DOI] [PubMed] [Google Scholar]
- Mannhold R., Rodenkirchen R., Bayer R., Haas W. The importance of drug ionization for the action of calcium-antagonists and related compounds. Arzneimittelforschung. 1984;34(4):407–409. [PubMed] [Google Scholar]
- Messing R. O., Carpenter C. L., Greenberg D. A. Inhibition of calcium flux and calcium channel antagonist binding in the PC12 neural cell line by phorbol esters and protein kinase C. Biochem Biophys Res Commun. 1986 May 14;136(3):1049–1056. doi: 10.1016/0006-291x(86)90439-0. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Feuvray D. Metabolic products and myocardial ischemia. Am J Pathol. 1981 Feb;102(2):282–291. [PMC free article] [PubMed] [Google Scholar]
- Osugi T., Imaizumi T., Mizushima A., Uchida S., Yoshida H. 1-Oleoyl-2-acetyl-glycerol and phorbol diester stimulate Ca2+ influx through Ca2+ channels in neuroblastoma x glioma hybrid NG108-15 cells. Eur J Pharmacol. 1986 Jul 15;126(1-2):47–51. doi: 10.1016/0014-2999(86)90736-3. [DOI] [PubMed] [Google Scholar]
- Palfreyman M. G., Mir A. K., Kubina M., Middlemiss D. N., Richards M., Tricklebank M. D., Fozard J. R. Monoamine receptor sensitivity changes following chronic administration of MDL 72394, a site-directed inhibitor of monoamine oxidase. Eur J Pharmacol. 1986 Oct 14;130(1-2):73–89. doi: 10.1016/0014-2999(86)90185-8. [DOI] [PubMed] [Google Scholar]
- Philipson K. D. Interaction of charged amphiphiles with Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. J Biol Chem. 1984 Nov 25;259(22):13999–14002. [PubMed] [Google Scholar]
- Philipson K. D., Langer G. A., Rich T. L. Charged amphiphiles regulate heart contractility and sarcolemma-Ca2+ interactions. Am J Physiol. 1985 Jan;248(1 Pt 2):H147–H150. doi: 10.1152/ajpheart.1985.248.1.H147. [DOI] [PubMed] [Google Scholar]
- Piper M. H., Sezer O., Schwartz P., Hütter J. F., Schweickhardt C., Spieckermann P. G. Acyl-carnitine effects on isolated cardiac mitochondria and erythrocytes. Basic Res Cardiol. 1984 Mar-Apr;79(2):186–198. doi: 10.1007/BF01908305. [DOI] [PubMed] [Google Scholar]
- Pitts B. J., Tate C. A., Van Winkle W. B., Wood J. M., Entman M. L. Palmitylcarnitine inhibition of the calcium pump in cardiac sarcoplasmic reticulum: a possible role in myocardial ischemia. Life Sci. 1978 Jul 24;23(4):391–401. doi: 10.1016/0024-3205(78)90025-5. [DOI] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Gould R. J., Snyder S. H. [3H]Verapamil binding sites in brain and skeletal muscle: regulation by calcium. Eur J Pharmacol. 1983 Nov 25;95(3-4):319–321. doi: 10.1016/0014-2999(83)90655-6. [DOI] [PubMed] [Google Scholar]
- Spedding M. Activators and inactivators of Ca++ channels: new perspectives. J Pharmacol. 1985 Oct-Dec;16(4):319–343. [PubMed] [Google Scholar]
- Spedding M. Assessment of "Ca2+ -antagonist" effects of drugs in K+ -depolarized smooth muscle. Differentiation of antagonist subgroups. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):234–240. doi: 10.1007/BF00500485. [DOI] [PubMed] [Google Scholar]
- Spedding M., Berg C. Interactions between a "calcium channel agonist", Bay K 8644, and calcium antagonists differentiate calcium antagonist subgroups in K+-depolarized smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1984 Nov;328(1):69–75. doi: 10.1007/BF00496109. [DOI] [PubMed] [Google Scholar]
- Spedding M. Changing surface charge with salicylate differentiates between subgroups of calcium-antagonists. Br J Pharmacol. 1984 Sep;83(1):211–220. doi: 10.1111/j.1476-5381.1984.tb10137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M. Competitive interactions between Bay K 8644 and nifedipine in K+ depolarized smooth muscle: a passive role for Ca2+? Naunyn Schmiedebergs Arch Pharmacol. 1985 Feb;328(4):464–466. doi: 10.1007/BF00692917. [DOI] [PubMed] [Google Scholar]
- Spedding M., Schini V., Schoeffter P., Miller R. C. Calcium channel activation does not increase release of endothelial-derived relaxant factors (EDRF) in rat aorta although tonic release of EDRF may modulate calcium channel activity in smooth muscle. J Cardiovasc Pharmacol. 1986 Nov-Dec;8(6):1130–1137. doi: 10.1097/00005344-198611000-00006. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Su C. M., Swamy V. C., Triggle D. J. Calcium channel activation in vascular smooth muscle by BAY K 8644. Can J Physiol Pharmacol. 1984 Nov;62(11):1401–1410. doi: 10.1139/y84-233. [DOI] [PubMed] [Google Scholar]
- Thomas G., Gross R., Schramm M. Calcium channel modulation: ability to inhibit or promote calcium influx resides in the same dihydropyridine molecule. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1170–1176. [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Wakade T. D. Phorbol ester facilitates 45Ca accumulation and catecholamine secretion by nicotine and excess K+ but not by muscarine in rat adrenal medulla. Nature. 1986 Jun 12;321(6071):698–700. doi: 10.1038/321698a0. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wise B. C., Kuo J. F. Modes of inhibition by acylcarnitines, adriamycin and trifluoperazine of cardiac phospholipid-sensitive calcium-dependent protein kinase. Biochem Pharmacol. 1983 Apr 1;32(7):1259–1265. doi: 10.1016/0006-2952(83)90280-0. [DOI] [PubMed] [Google Scholar]
- de Leiris J., Feuvray D. Ischaemia-induced damage in the working rat heart preparation: the effect of perfusate substrate composition upon subendocardial ultrastructure of the ischaemic left ventricular wall. J Mol Cell Cardiol. 1977 May;9(5):365–373. doi: 10.1016/s0022-2828(77)80003-5. [DOI] [PubMed] [Google Scholar]


