Abstract
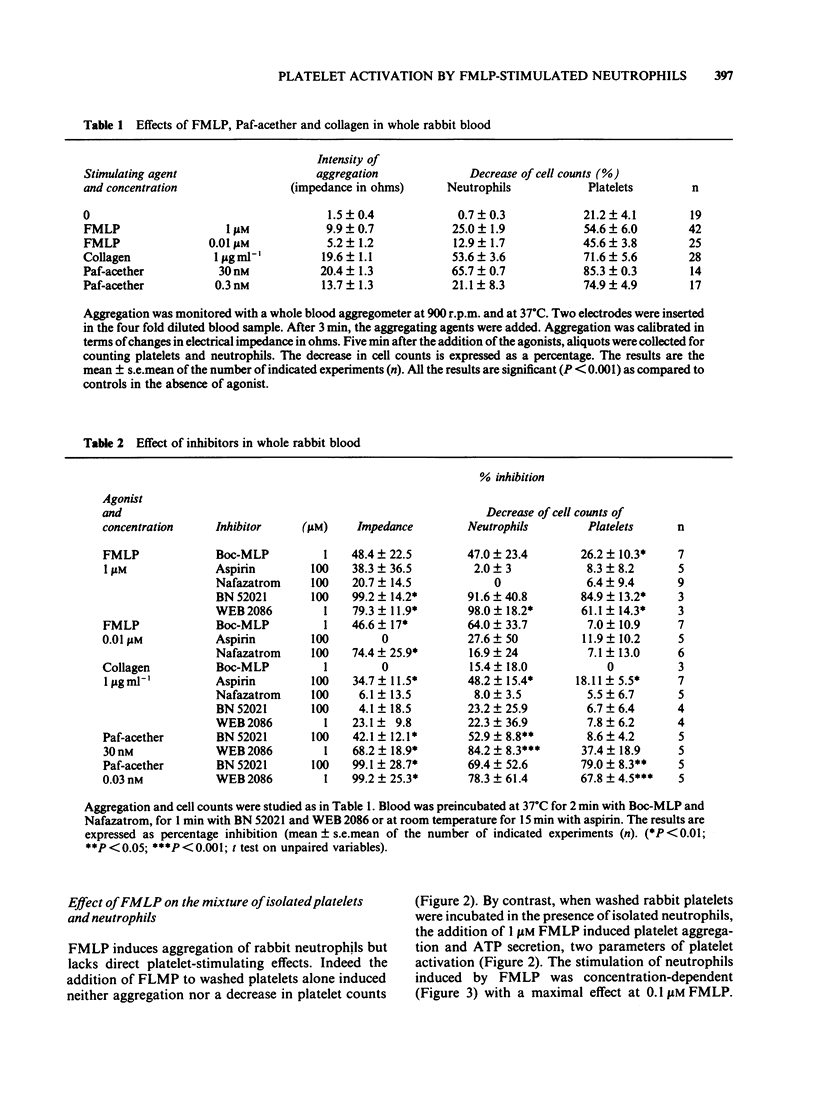
1 The effect of the chemotactic peptide, N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP) was studied on cells in whole rabbit blood or on a mixture of purified rabbit platelets and neutrophils. 2 In blood, FMLP triggered cell aggregation (measured by electrical impedance) which was dependent upon the concentration of FMLP (9.9 +/- 0.7 and 5.2 +/- 1.2 ohms at 1 and 0.01 microM FMLP respectively). This aggregation was accompanied by a strong decrease in platelet counts (54.6 +/- 6.0 and 45.6 +/- 3.8% for 1 and 0.01 microM FMLP respectively) and by a smaller decrease in neutrophil counts (25.0 +/- 1.9 and 12.9 +/- 1.7% at 1 and 0.01 microM FMLP respectively). 3 When purified platelets and neutrophils were co-incubated, the addition of 0.1 microM induced a marked aggregation (50.0 +/- 1.6 vs. 19.5 +/- 1.6% of light transmission, n = 8, P less than 0.001), ATP secretion (8.4 +/- 1.0 vs. 0.1 +/- 0.1 nmol ml-1, n = 6, P less than 0.001) and a decrease in platelet counts. FMLP induced aggregation of purified neutrophils and release of lysozyme but lacked direct platelet-stimulating effects. The release of lactate dehydrogenase, a cytoplasmic marker and lysozyme were unchanged under the interaction conditions. 4 Platelet activation was reduced by about 30% with 100 microM aspirin or indomethacin and by about 70% with 100 microM BW 755C. Two Paf-acether antagonists, BN 52021 (100 microM) and WEB 2086 (1 microM) suppressed platelet activation by 70-80%. 5 The supernatant of FMLP-stimulated neutrophils induced platelet activation only when bovine serum albumin was present. Rabbit neutrophils stimulated in the presence of serum albumin by 1 microM FMLP formed 2 nM Paf-acether of which half was released to the extracellular medium. 6 Our results indicate that the stimulation of neutrophils by FMLP induces platelet activation in whole blood and on isolated cells and that both arachidonic acid-metabolites and Paf-acether participate in platelet activation.
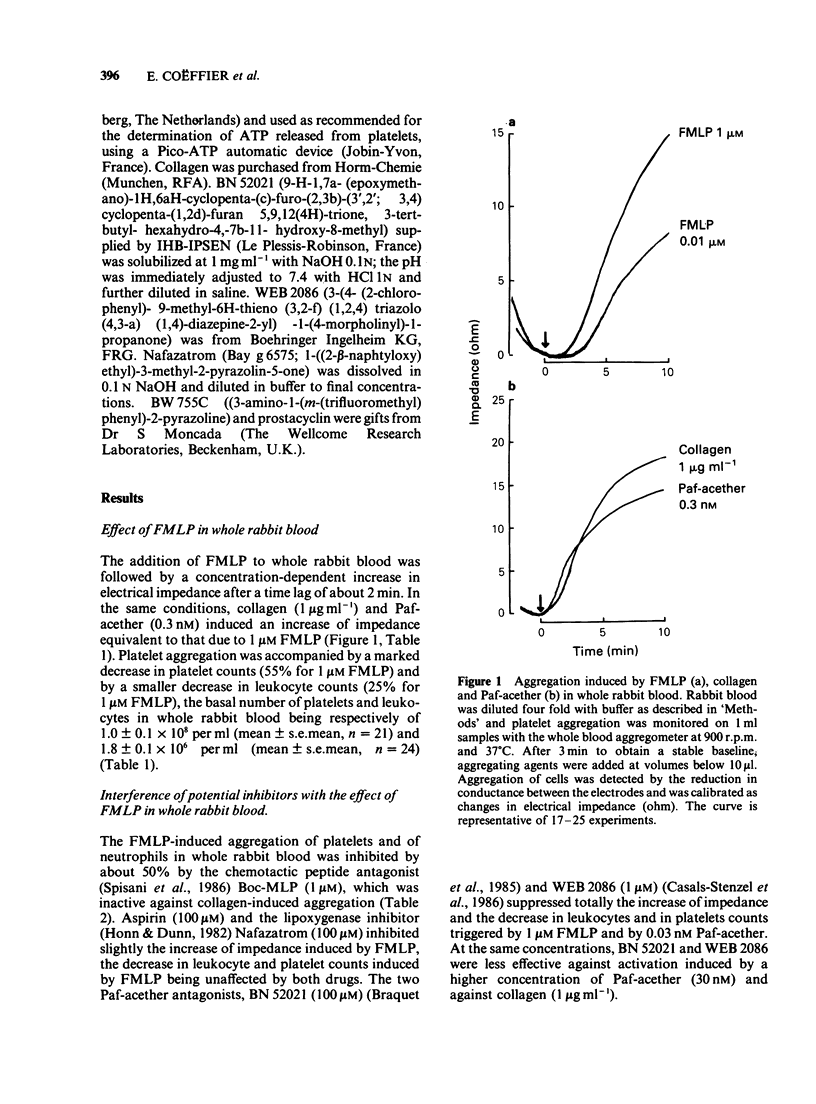
Full text
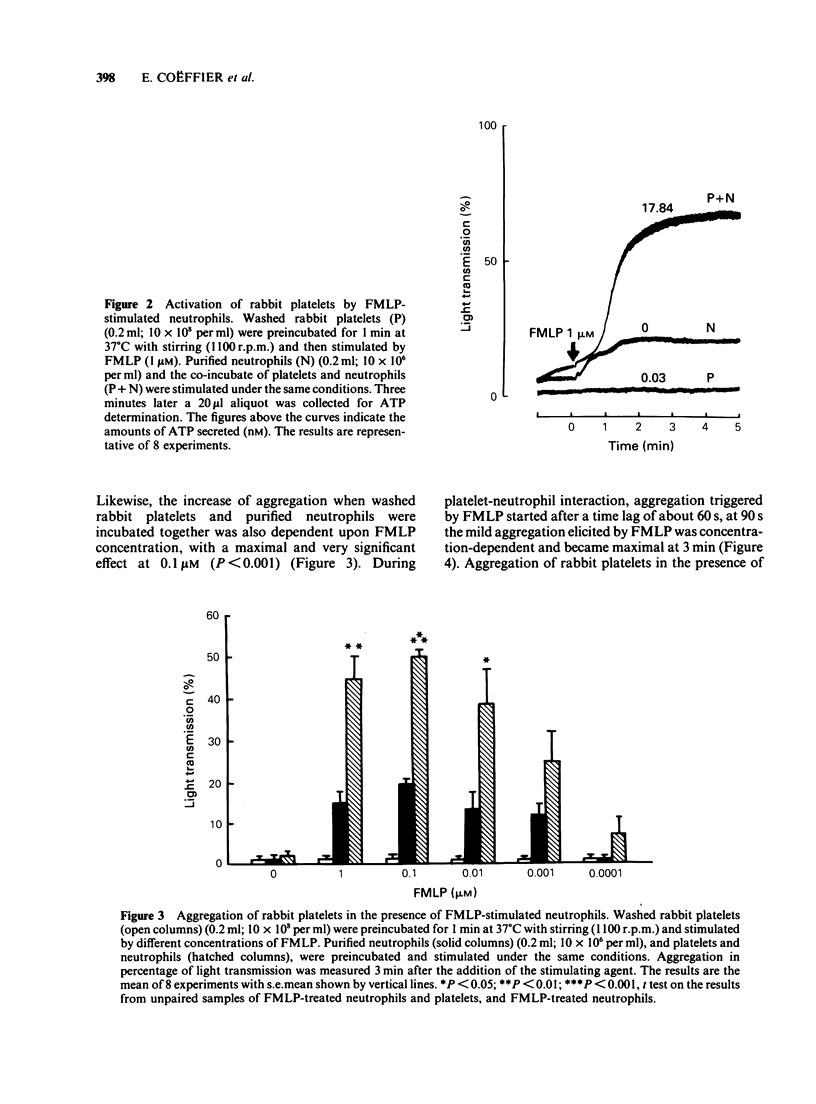
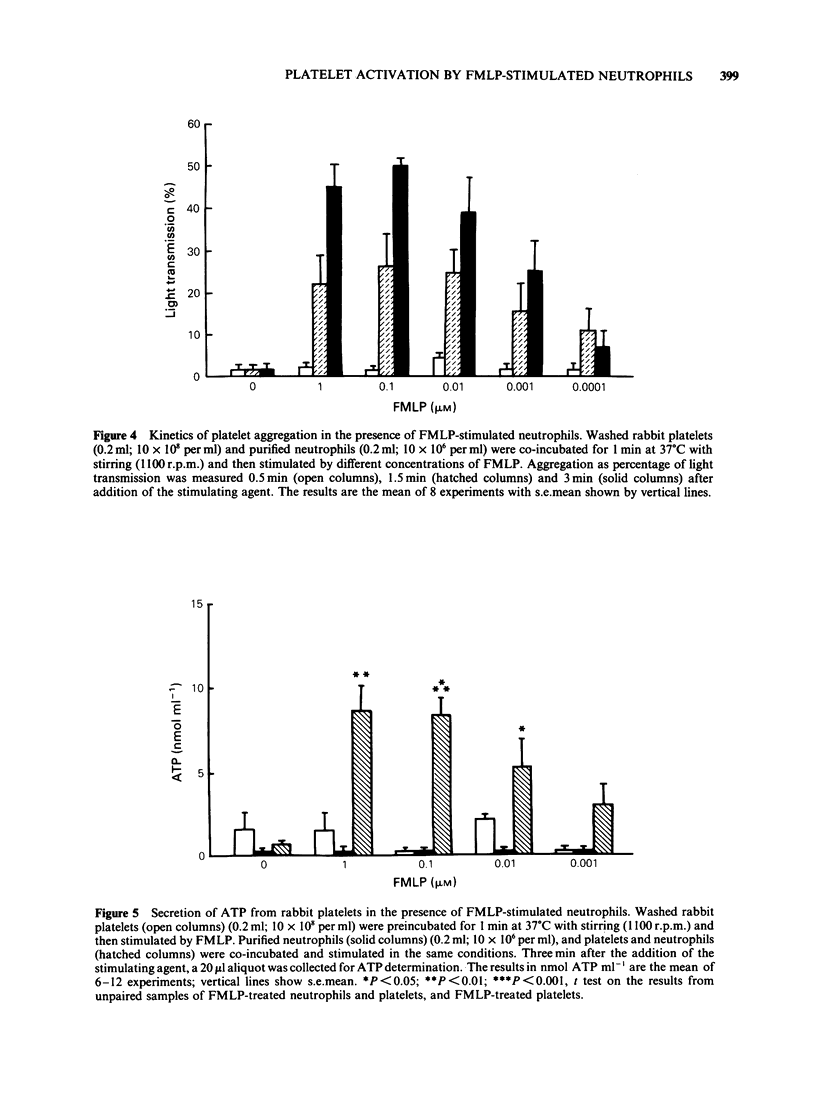
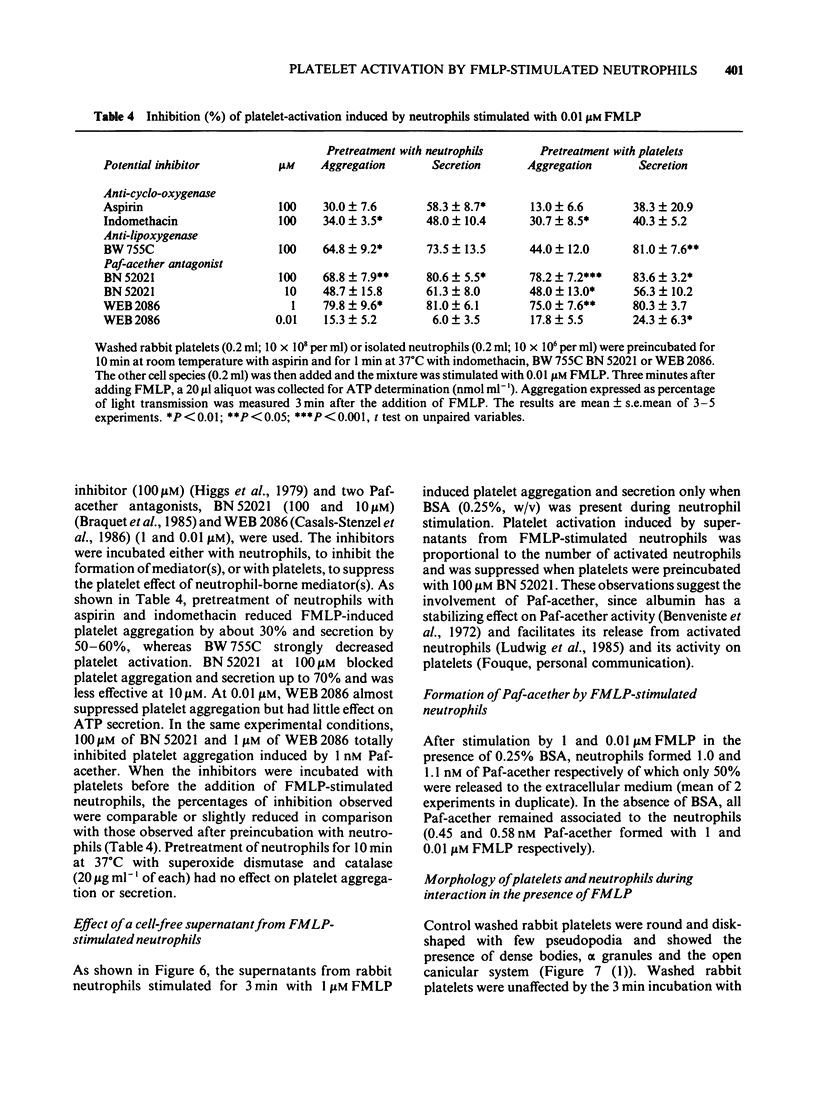
PDF



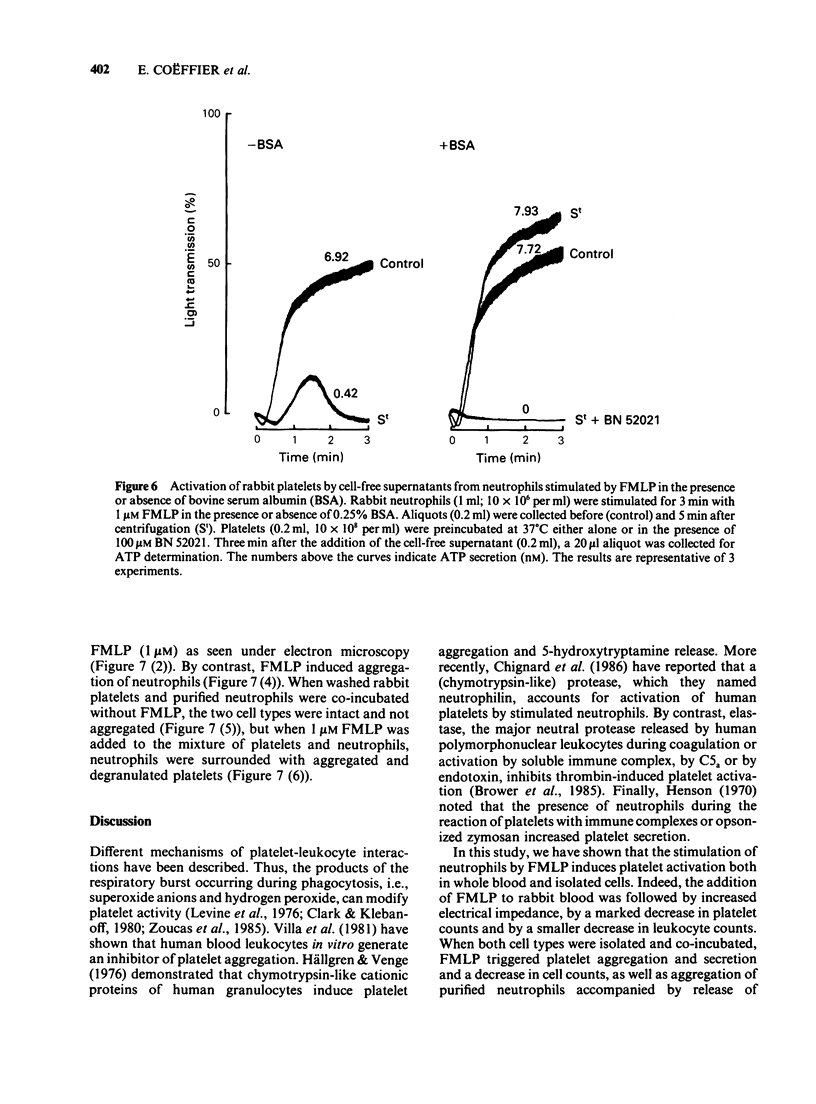
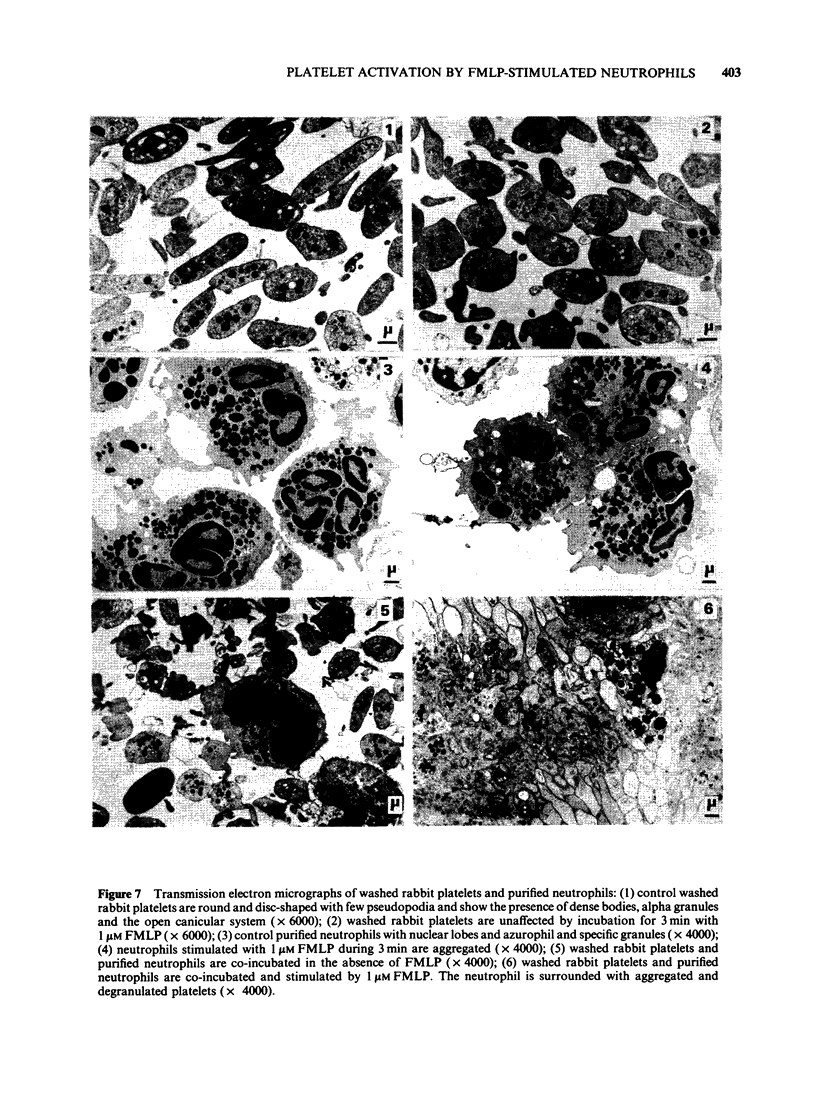



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Berend N., Armour C. L., Black J. L. Formyl-methionyl-leucyl-phenylalanine causes bronchoconstriction in rabbits. Agents Actions. 1986 Mar;17(5-6):466–471. doi: 10.1007/BF01965515. [DOI] [PubMed] [Google Scholar]
- Boukili M. A., Bureau M., Lagente V., Lefort J., Lellouch-Tubiana A., Malanchère E., Vargaftig B. B. Pharmacological modulation of the effects of N-formyl-L-methionyl-L-leucyl-L-phenylalanine in guinea-pigs: involvement of the arachidonic acid cascade. Br J Pharmacol. 1986 Oct;89(2):349–359. doi: 10.1111/j.1476-5381.1986.tb10267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgain R. H., Maes L., Braquet P., Andries R., Touqui L., Braquet M. The effect of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (paf-acether) on the arterial wall. Prostaglandins. 1985 Aug;30(2):185–197. doi: 10.1016/0090-6980(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Brindley L. L., Sweet J. M., Goetzl E. J. Stimulation of histamine release from human basophils by human platelet factor 4. J Clin Invest. 1983 Oct;72(4):1218–1223. doi: 10.1172/JCI111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower M. S., Levin R. I., Garry K. Human neutrophil elastase modulates platelet function by limited proteolysis of membrane glycoproteins. J Clin Invest. 1985 Feb;75(2):657–666. doi: 10.1172/JCI111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignard M., Selak M. A., Smith J. B. Direct evidence for the existence of a neutrophil-derived platelet activator (neutrophilin). Proc Natl Acad Sci U S A. 1986 Nov;83(22):8609–8613. doi: 10.1073/pnas.83.22.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-platelet interaction mediated by myeloperoxidase and hydrogen peroxide. J Immunol. 1980 Jan;124(1):399–405. [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Falloon J., Gallin J. I. Neutrophil granules in health and disease. J Allergy Clin Immunol. 1986 May;77(5):653–662. doi: 10.1016/0091-6749(86)90404-5. [DOI] [PubMed] [Google Scholar]
- HENRY R. L. LEUKOCYTES AND THROMBOSIS. Thromb Diath Haemorrh. 1965 Mar 15;13:35–46. [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of release of constituents from rabbit platelets by antigen-antibody complexes and complement. II. Interaction of platelets with neutrophils. J Immunol. 1970 Aug;105(2):490–501. [PubMed] [Google Scholar]
- Higgs G. A., Bunting S., Moncada S., Vane J. R. Polymorphonuclear leukocytes produce thromboxane A2-like activity during phagocytosis. Prostaglandins. 1976 Nov;12(5):749–757. doi: 10.1016/0090-6980(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Flower R. J., Vane J. R. A new approach to anti-inflammatory drugs. Biochem Pharmacol. 1979 Jun 15;28(12):1959–1961. doi: 10.1016/0006-2952(79)90651-8. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Further evidence for a deficient storage pool of adenine nucleotides in platelets from some patients with thrombocytopathia--"storage pool disease". Blood. 1972 Feb;39(2):197–209. [PubMed] [Google Scholar]
- Honn K. V., Dunn J. R. Nafazatrom (Bay g 6575) inhibition of tumor cell lipoxygenase activity and cellular proliferation. FEBS Lett. 1982 Mar 8;139(1):65–68. doi: 10.1016/0014-5793(82)80488-2. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Jackson J. R. Role of neutrophils in the deposition of platelets during acute inflammation. Lab Invest. 1983 Dec;49(6):716–724. [PubMed] [Google Scholar]
- Jouvin-Marche E., Ninio E., Beaurain G., Tence M., Niaudet P., Benveniste J. Biosynthesis of Paf-acether (platelet-activating factor). VII. Precursors of Paf-acether and acetyl-transferase activity in human leukocytes. J Immunol. 1984 Aug;133(2):892–898. [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Dougherty H. W., Ham E. A. Interactions between prostaglandins and leukotrienes. Biochem Pharmacol. 1984 Jan 1;33(1):1–5. doi: 10.1016/0006-2952(84)90362-9. [DOI] [PubMed] [Google Scholar]
- LITWACK G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955 Jul;89(3):401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- Levine P. H., Weinger R. S., Simon J., Scoon K. L., Krinsky N. I. Leukocyte-platelet interaction. Release of hydrogen peroxide by granulocytes as a modulator of platelet reactions. J Clin Invest. 1976 Apr;57(4):955–963. doi: 10.1172/JCI108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J. C., Hoppens C. L., McManus L. M., Mott G. E., Pinckard R. N. Modulation of platelet-activating factor (PAF) synthesis and release from human polymorphonuclear leukocytes (PMN): role of extracellular albumin. Arch Biochem Biophys. 1985 Sep;241(2):337–347. doi: 10.1016/0003-9861(85)90555-7. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Henson P. M. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986 Oct 15;137(8):2653–2661. [PubMed] [Google Scholar]
- Lynch J. M., Lotner G. Z., Betz S. J., Henson P. M. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Broekman M. J., Safier L. B., Ullman H. L., Islam N., Sherhan C. N., Rutherford L. E., Korchak H. M., Weissmann G. Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem Biophys Res Commun. 1982 Nov 16;109(1):130–137. doi: 10.1016/0006-291x(82)91575-3. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Broekman M. J., Islam N., Oglesby T. D., Gorman R. R. 12S,20-dihydroxyicosatetraenoic acid: a new icosanoid synthesized by neutrophils from 12S-hydroxyicosatetraenoic acid produced by thrombin- or collagen-stimulated platelets. Proc Natl Acad Sci U S A. 1984 Feb;81(3):903–907. doi: 10.1073/pnas.81.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Islam N., Broekman M. J., von Schacky C. Studies on the mechanism of omega-hydroxylation of platelet 12-hydroxyeicosatetraenoic acid (12-HETE) by unstimulated neutrophils. J Clin Invest. 1987 Jan;79(1):179–187. doi: 10.1172/JCI112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Satouchi K., Yasunaga K., Saito K. Polymorphonuclear leukocyte-platelet interactions: acetylglyceryl ether phosphocholine-induced platelet activation under stimulation with chemotactic peptide. J Biochem. 1986 Nov;100(5):1117–1123. doi: 10.1093/oxfordjournals.jbchem.a121815. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Sklar L. A., Jesaitis A. J., Schmitt M., Cochrane C. G. Activation of neutrophils by N-formyl chemotactic peptides. Fed Proc. 1984 Sep;43(12):2737–2742. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Smith N. C., Flower R. J., Cardinal D. C. Measuring platelet and leucocyte aggregation/adhesion responses in very small volumes of whole blood. J Pharmacol Methods. 1981 Dec;6(4):315–333. doi: 10.1016/0160-5402(81)90071-1. [DOI] [PubMed] [Google Scholar]
- Spisani S., Cavalletti T., Gavioli R., Scatturin A., Vertuani G., Traniello S. Response of human neutrophils to formyl-peptide modified at the terminal amino and carboxyl groups. Inflammation. 1986 Dec;10(4):363–369. doi: 10.1007/BF00915820. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Villa S., Rotilio D., Donati M. B., de Gaetano G. Human blood leucocytes in vitro generate an inhibitor of platelet aggregation. Thromb Res. 1981 Dec 1;24(5-6):485–487. doi: 10.1016/0049-3848(81)90083-9. [DOI] [PubMed] [Google Scholar]
- Zoucas E., Fäldt R., Ankerst J. Effect of latex-stimulated granulocytes on platelet aggregation in man. Haemostasis. 1985;15(3):176–181. doi: 10.1159/000215141. [DOI] [PubMed] [Google Scholar]