Abstract
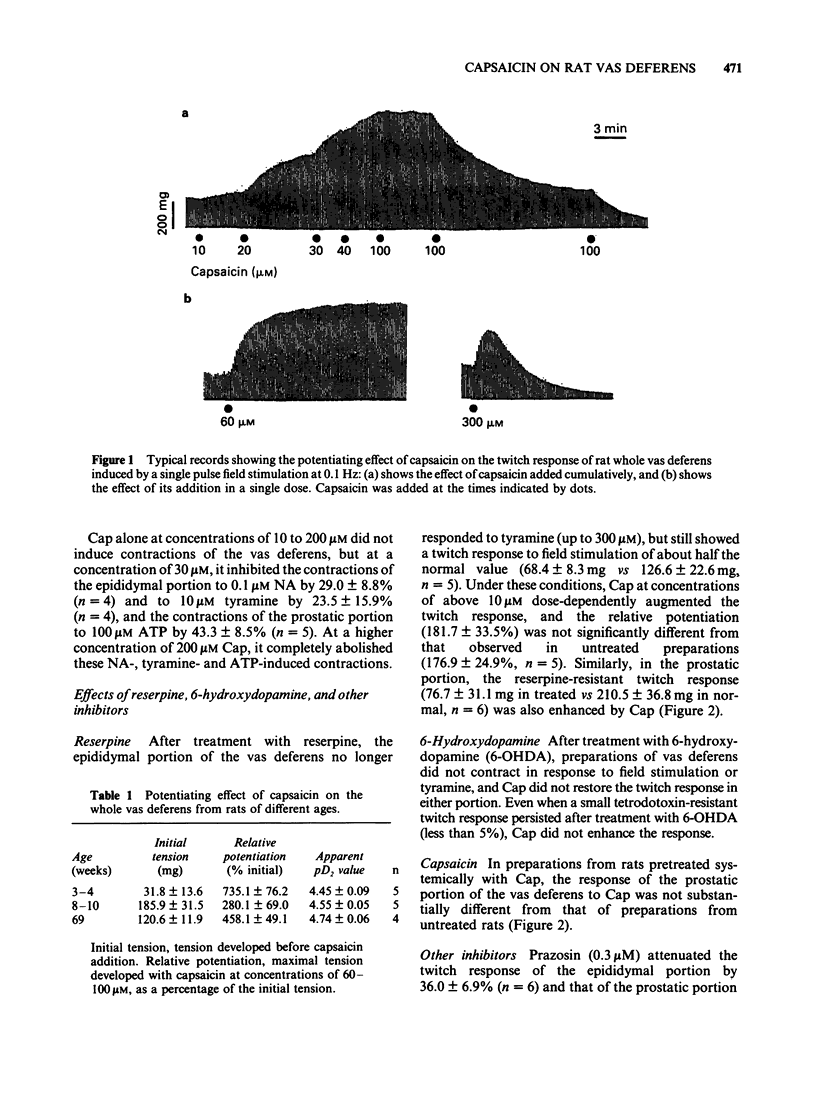
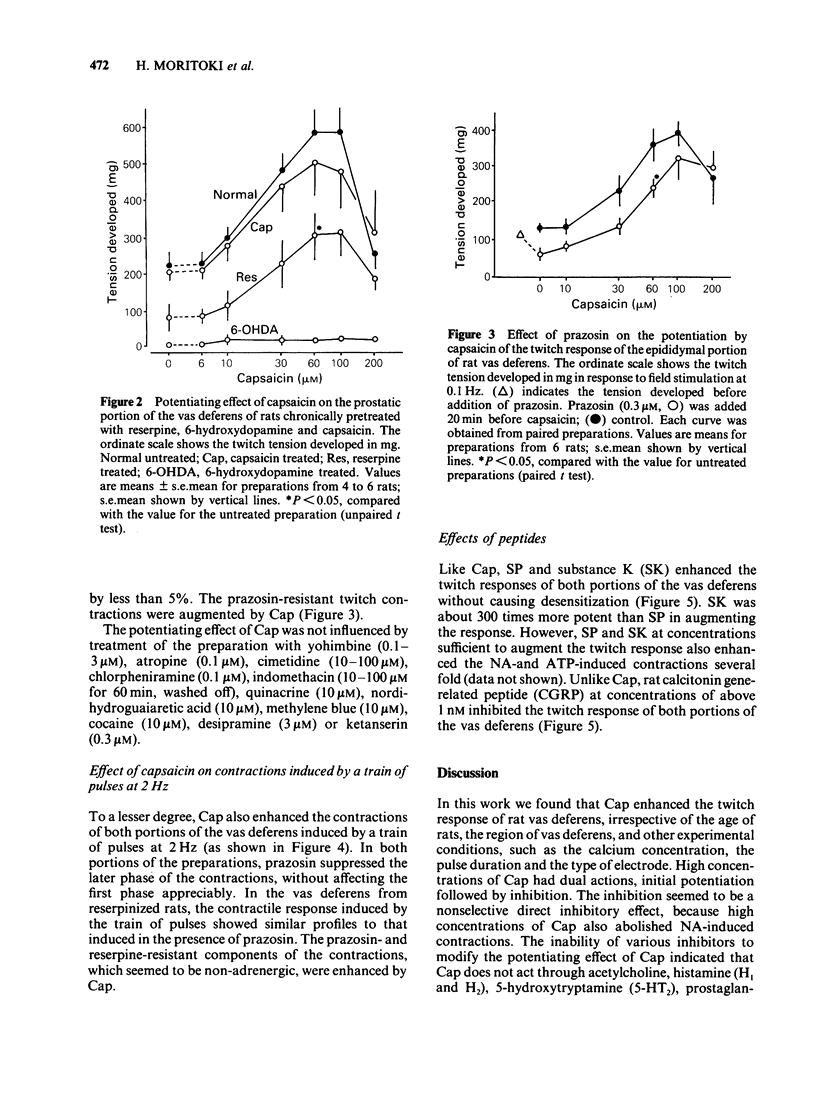
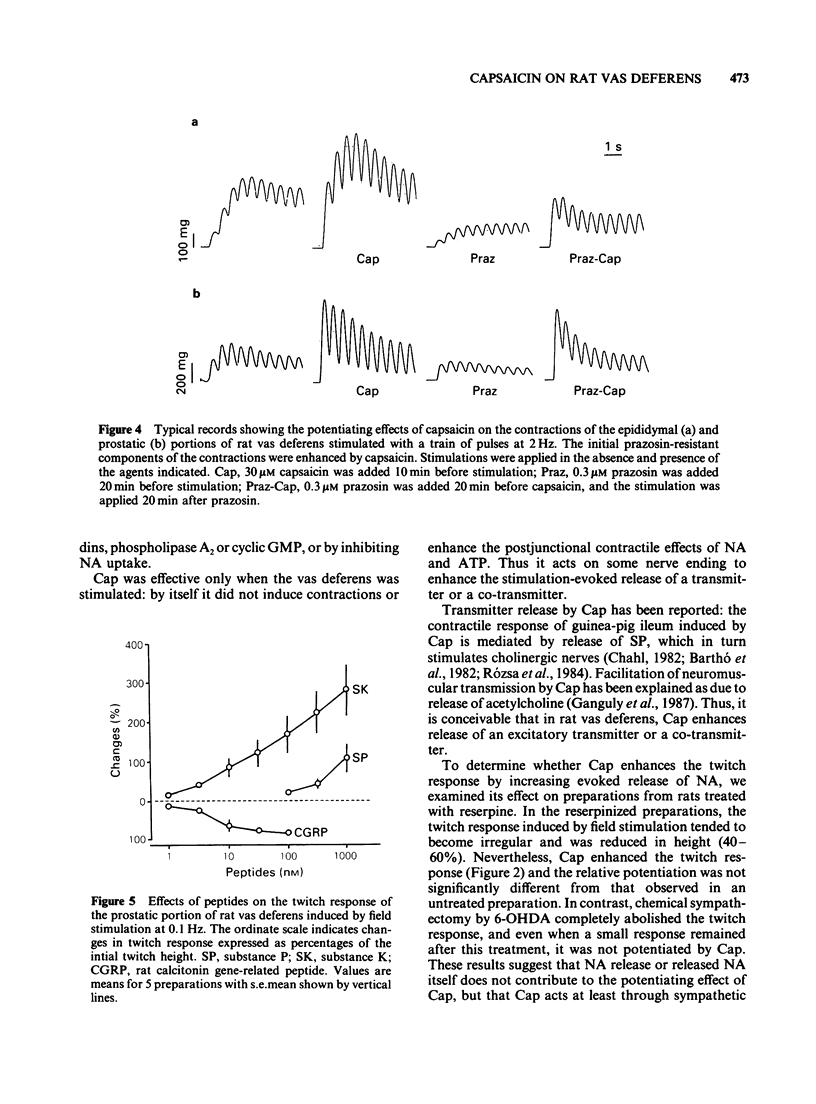
1 Capsaicin (Cap) enhanced the twitch response of the epididymal and prostatic portions of rat vas deferens induced by field stimulation at 0.1 Hz. The effect of Cap was reproducible and showed no desensitization. 2 Prazosin, and pretreatment with reserpine or Cap did not affect the potentiating effect of Cap, whereas pretreatment with 6-hydroxydopamine abolished the action of Cap. 3 Cap tended to attenuate the contractions induced by noradrenaline, tyramine and ATP. 4 Like Cap, substance K and substance P augmented the twitch response without causing desensitization, but their effects differed somewhat from that of Cap. Calcitonin gene-related peptide inhibited the twitch response. 5 These results suggest that Cap enhances a stimulation-induced, prazosin-resistant non-adrenergic twitch response of rat vas deferens through an as yet undefined prejunctional mechanism. This mechanism is possibly mediated by some peptide released in response to Cap from sensory neurones, which in turn acts on sympathetic nerves and increases stimulation-induced release of a mediator or cotransmitter responsible for the non-adrenergic twitch response. However, the possibility that Cap has a direct action on sympathetic nerves cannot be ruled out.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Kitagawa K., Kiyama S., Kawamoto T., Akita T., Yamamoto I., Torizuka K. Synthesis of a 37-residue peptide amide corresponding to the entire amino acid sequence of alpha-form of rat calcitonin gene-related peptide (alpha-rCGRP). Chem Pharm Bull (Tokyo) 1987 Jan;35(1):60–71. doi: 10.1248/cpb.35.60. [DOI] [PubMed] [Google Scholar]
- Barthó L., Holzer P., Lembeck F., Szolcsányi J. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to release of substance P. J Physiol. 1982 Nov;332:157–167. doi: 10.1113/jphysiol.1982.sp014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahl L. A. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to substance P release. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):212–215. doi: 10.1007/BF00495867. [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Buck S. H. Substance P in the cerebral vasculature: depletion by capsaicin suggests a sensory role. Brain Res. 1982 Aug 5;245(1):171–174. doi: 10.1016/0006-8993(82)90355-9. [DOI] [PubMed] [Google Scholar]
- French A. M., Scott N. C. Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia. 1983 Mar 15;39(3):264–266. doi: 10.1007/BF01955295. [DOI] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Ganguly D. K., Das M., Das Gupta A. K., Chauhan S. P. Possible functional role of substance P on the mammalian motor nerve terminals. Life Sci. 1987 Jan 19;40(3):289–292. doi: 10.1016/0024-3205(87)90345-6. [DOI] [PubMed] [Google Scholar]
- Hanko J., Hardebo J. E., Kåhrström J., Owman C., Sundler F. Existence and coexistence of calcitonin gene-related peptide (CGRP) and substance P in cerebrovascular nerves and trigeminal ganglion cells. Acta Physiol Scand Suppl. 1986;552:29–32. [PubMed] [Google Scholar]
- Holzer P., Gamse R., Lembeck F. Distribution of substance P in the rat gastrointestinal tract--lack of effect of capsaicin pretreatment. Eur J Pharmacol. 1980 Feb 8;61(3):303–307. doi: 10.1016/0014-2999(80)90132-6. [DOI] [PubMed] [Google Scholar]
- Hua X. Y., Lundberg J. M. Dual capsaicin effects on ureteric motility: low dose inhibition mediated by calcitonin gene-related peptide and high dose stimulation by tachykinins? Acta Physiol Scand. 1986 Nov;128(3):453–465. doi: 10.1111/j.1748-1716.1986.tb07999.x. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Franco-Cereceda A., Hua X., Hökfelt T., Fischer J. A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985 Feb 5;108(3):315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hua Y., Fredholm B. B. Capsaicin-induced stimulation of the guinea-pig atrium. Involvement of a novel sensory transmitter or a direct action on myocytes? Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):176–182. doi: 10.1007/BF00506198. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Manzini S., Giuliani S., Santicioli P., Meli A. Extrinsic origin of the capsaicin-sensitive innervation of rat duodenum: possible involvement of calcitonin gene-related peptide (CGRP) in the capsaicin-induced activation of intramural non-adrenergic non-cholinergic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1986 Oct;334(2):172–180. doi: 10.1007/BF00505818. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Giuliani S., Abelli L., Meli A. The motor effect of the capsaicin-sensitive inhibitory innervation of the rat ureter. Eur J Pharmacol. 1986 Jul 31;126(3):333–336. doi: 10.1016/0014-2999(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Manzini S., Meli A. Capsaicin activates neurogenic non-adrenergic non-cholinergic relaxations of the isolated rat duodenum. Eur J Pharmacol. 1986 Jan 29;120(3):367–370. doi: 10.1016/0014-2999(86)90480-2. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Meli A. Evidence for the involvement of endogenous substance P in the motor effects of capsaicin on the rat urinary bladder. J Pharm Pharmacol. 1985 Mar;37(3):203–204. doi: 10.1111/j.2042-7158.1985.tb05042.x. [DOI] [PubMed] [Google Scholar]
- Nawa H., Doteuchi M., Igano K., Inouye K., Nakanishi S. Substance K: a novel mammalian tachykinin that differs from substance P in its pharmacological profile. Life Sci. 1984 Mar 19;34(12):1153–1160. doi: 10.1016/0024-3205(84)90087-0. [DOI] [PubMed] [Google Scholar]
- Rózsa Z., Jancsó G., Varró V. Possible involvement of capsaicin-sensitive sensory nerves in the regulation of intestinal blood flow in the dog. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jul;326(4):352–356. doi: 10.1007/BF00501442. [DOI] [PubMed] [Google Scholar]
- Rózsa Z., Varró A., Jancsó G. Use of immunoblockade to study the involvement of peptidergic afferent nerves in the intestinal vasodilatory response to capsaicin in the dog. Eur J Pharmacol. 1985 Sep 10;115(1):59–64. doi: 10.1016/0014-2999(85)90584-9. [DOI] [PubMed] [Google Scholar]
- Saito A., Goto K. Depletion of calcitonin gene-related peptide (CGRP) by capsaicin in cerebral arteries. J Pharmacobiodyn. 1986 Jul;9(7):613–619. doi: 10.1248/bpb1978.9.613. [DOI] [PubMed] [Google Scholar]
- Saito A., Kimura S., Goto K. Calcitonin gene-related peptide as potential neurotransmitter in guinea pig right atrium. Am J Physiol. 1986 Apr;250(4 Pt 2):H693–H698. doi: 10.1152/ajpheart.1986.250.4.H693. [DOI] [PubMed] [Google Scholar]
- Santicioli P., Maggi C. A., Meli A. Functional evidence for the existence of a capsaicin-sensitive innervation in the rat urinary bladder. J Pharm Pharmacol. 1986 Jun;38(6):446–451. doi: 10.1111/j.2042-7158.1986.tb04608.x. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Colby J., Fedan J. S. A pharmacological investigation of the biphasic nature of the contractile response of rabbit and rat vas deferens to field stimulation. Life Sci. 1984 Nov 5;35(19):1903–1912. doi: 10.1016/0024-3205(84)90470-3. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J., Barthó L. Capsaicin-sensitive innervation of the guinea-pig taenia caeci. Naunyn Schmiedebergs Arch Pharmacol. 1979 Oct;309(1):77–82. doi: 10.1007/BF00498759. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J., Barthó L. Capsaicin-sensitive non-cholinergic excitatory innervation of the guinea-pig tracheobronchial smooth muscle. Neurosci Lett. 1982 Dec 31;34(3):247–251. doi: 10.1016/0304-3940(82)90183-5. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Bynke G., Håkanson R. Pupillary constriction by bradykinin and capsaicin: mode of action. Eur J Pharmacol. 1984 Nov 27;106(3):577–583. doi: 10.1016/0014-2999(84)90061-x. [DOI] [PubMed] [Google Scholar]


