Abstract
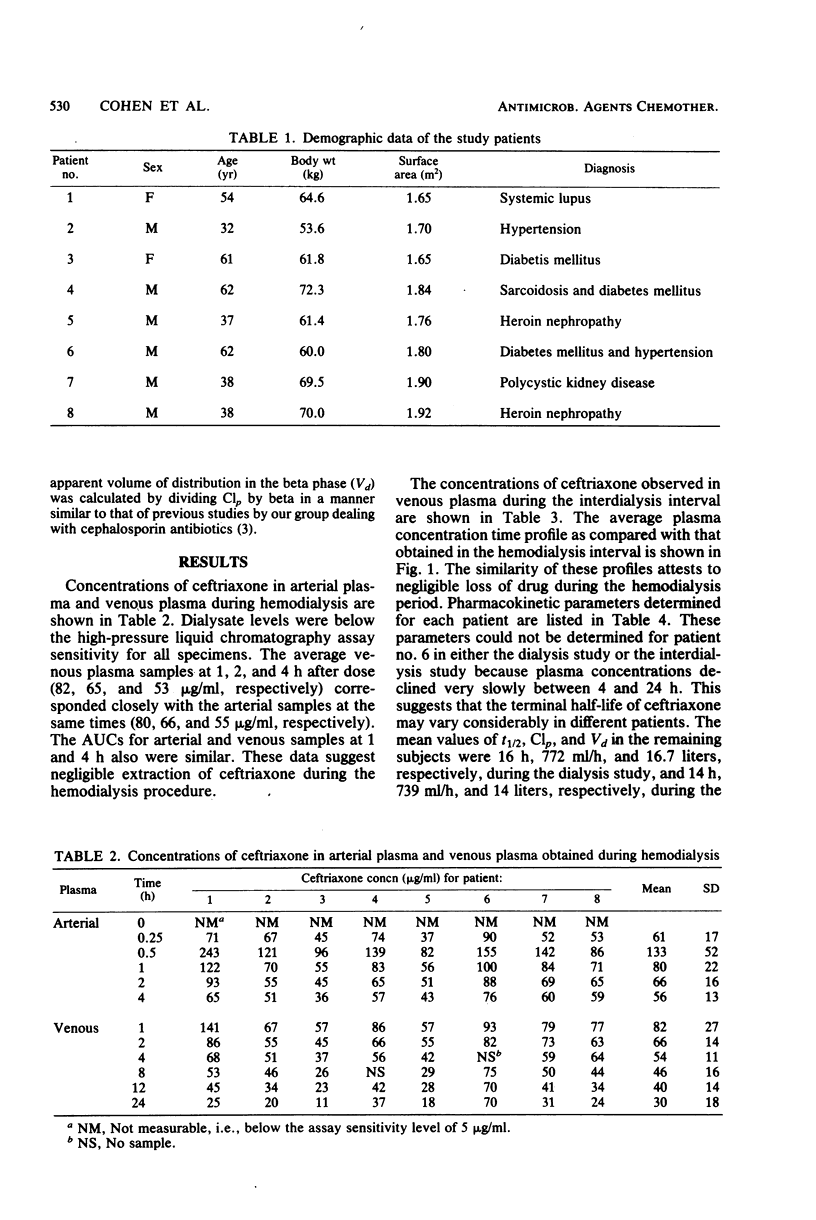
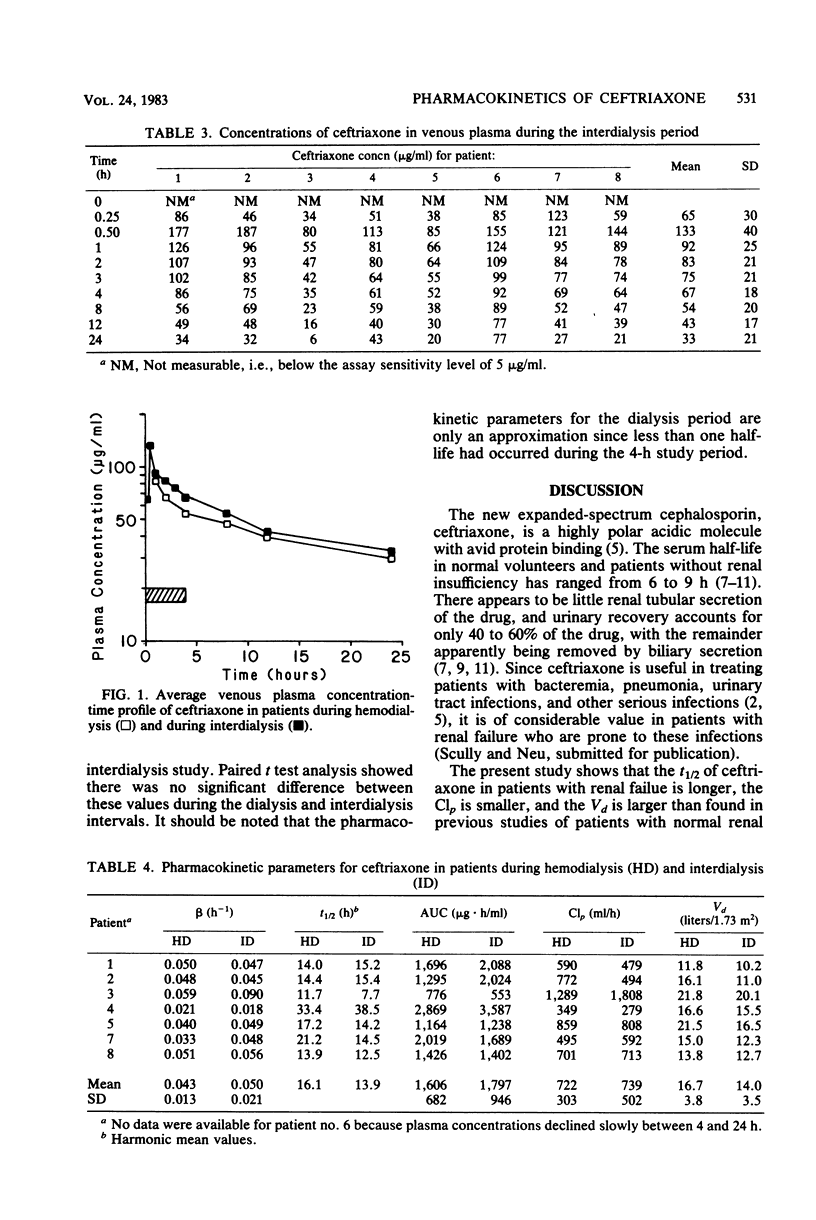
The pharmacokinetic parameters of ceftriaxone in eight patients with end-stage renal disease were determined during dialysis and during the interdialysis period. The mean half-life, clearance, and apparent volume of distribution during dialysis were 16 h, 722 ml/h, and 16.7 liters, respectively. During the interdialysis period, the half-life was 14 h, clearance was 739 ml/h, and volume of distributions was 14 liters. Individual variability in plasma concentrations occurred even in patients with apparently normal hepatic function. Based on these parameters, a dose of 1 g every 24 h would yield concentrations in excess of the concentrations needed to inhibit most gram-positive and gram-negative aerobic species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosen V., Chamali R., Bar-Moshe O., Stenier P. Clinical study of Rocephin, a 3d generation cephalosporin, in various septicaemias. Chemotherapy. 1981;27 (Suppl 1):100–103. doi: 10.1159/000238036. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Miller K., Weinfeld R., Spicehandler J. Multiple intravenous dose pharmacokinetics of ceftriaxone in man. Chemotherapy. 1981;27 (Suppl 1):47–56. doi: 10.1159/000238029. [DOI] [PubMed] [Google Scholar]
- Pollock A. A., Tee P. E., Patel I. H., Spicehandler J., Simberkoff M. S., Rahal J. J., Jr Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother. 1982 Nov;22(5):816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K. Pharmacokinetics of Rocephin, a highly active new cephalosporin with an exceptionally long biological half-life. Chemotherapy. 1981;27 (Suppl 1):42–46. doi: 10.1159/000238028. [DOI] [PubMed] [Google Scholar]



