Abstract
Many of the effects of estrogens on the uterus are mediated by ERα, the predominant ER in the mature organ. Because of the poor reproductive capacity of ERβ knockout (BERKO) female mice (small litter size, multiple-resorbed fetuses), the role of uterine ERβ was explored. In the immature uterus, ERα and ERβ are expressed at comparable levels in the epithelium and stroma, and 17β-estradiol (E2) treatment decreases ERβ in the stroma. The immature uterus of untreated BERKO mice exhibits elevated levels of progesterone receptor (PR) and the proliferation-associated protein, Ki-67. It also exhibits exaggerated responsiveness to E2, as indicated by enlargement of the lumen, increase in volume and protein content of uterine secretion, induction of the luminal epithelial secretory protein, complement C3, and its regulatory cytokine IL-1β, and induction of vascular endothelial growth factor and insulin-like growth factor 1 but not its receptor. As expected, E2 increased PR in the stroma and decreased it in the luminal epithelium of wild-type mice. In the BERKO uterus, E2 induced PR in the stroma but did not down-regulate it in the epithelium. Increased cell proliferation and exaggerated response to E2 in BERKO suggest that ERβ plays a role in modulation of the effects of ERα and in addition (or as a consequence of this) has an antiproliferative function in the immature uterus.
The transcriptional effects of 17β-estradiol (E2) in the body are mediated by two distinct ERs, ERα and ERβ. A definitive role for ERα in the uterotrophic effects of E2 was confirmed in adult female ERα knockout mice, where there is loss of estrogen responsiveness (1), as well as in mice with disruption of the estrogen-responsive ring finger protein gene (2). ERβ is present in both endometrium and myometrium in several animal species (3–8), but its function in the uterus remains unknown.
The uterus, a major target tissue for ovarian hormones, is composed of heterogeneous cell types (stroma, luminal epithelial, glandular epithelial, and smooth muscle), that undergo continuous synchronized changes of proliferation and differentiation in response to changes in levels of circulating estrogen and progesterone (9, 10). Estrogen, by regulating estrogen target genes in a cell-specific manner, has different effects on different types of cells in the uterus. In the immature (21-day-old) mouse uterus, the cellular proliferation rate is very low (9). Proliferation is initiated at puberty in response to cycling estrogen, although the immature uterus is fully capable of responding to E2, and estrogen induces both epithelial and stromal cell proliferation (10). Although ERα is highly expressed in the epithelium, proliferation of epithelial cells in response to E2 is thought to be indirect, i.e., growth factors secreted by stroma in response to estrogen are the mitogens in the epithelium (11). ERα plays an important role in differentiation by regulating target genes such as that for the progesterone receptor (PR). Both the stroma and epithelium express PR, and estrogen induces PR in the stromal and glandular epithelial cells, whereas it reduces it in the luminal epithelial cells (12). The mechanism behind the cell-specific regulation of PR by estrogen remains unclear and cannot be fully explained if all of the actions of estrogen in the uterus are mediated by ERα. In vitro studies have shown that, depending on the nature of ligand and response elements on DNA with which the activated receptors interact, ERα and ERβ can have opposite effects on gene transcription (13). ERβ can act as a transdominant repressor on ERα transcriptional activity at subsaturating concentrations of E2 (14). One explanation for the inhibitory effects of ERβ on ERα function is that ERβ can form heterodimers with ERα (15, 16), which in turn regulate ER functions. If this were the case, ERβ would not be expected to have classic uterotrophic effects but would rather dampen the effects on ERα.
ERβ levels in the uterus change during the menstrual cycle with the highest levels present during the proliferation phase (3) when estrogen and ERα levels are also at their peaks. To explore the role of ERβ in the uterus, we examined the uterine morphology and estrogen regulation of gene expression in wild-type and ERβ knockout (BERKO) mice. We found that ERβ acts as modulator of ERα-mediated gene transcription in the uterus, and furthermore, that it is responsible for down-regulation of PR in the luminal epithelium.
Materials and Methods
Animals.
Wild-type and BERKO female mice (21-day-old) were used. 17β-Estradiol dissolved in Intralipid (Pharmacia) was administered by s.c. injection for 3 days at a dose of 50 μg/kg/day. The control group was given the same volume of vehicle. Animals were asphyxiated by CO2, and the reproductive tracts were collected and photographed. Immediately after photography, the uterine fluid from one horn was collected by aspiration. The other horn was either frozen in liquid nitrogen for RNA analysis and ERβ immunohistochemical staining or put into 4% paraformaldehyde for histological evaluation and Ki-67, PR, insulin-like growth factor 1 receptor (IGF-1R), and vascular endothelial growth factor (VEGF) immunohistochemical analysis.
Chemicals and Antibodies.
17β-Estradiol was from Sigma. Polyclonal antibodies raised against PR (C-19, rabbit), Ki-67 (M-19, goat), IGF-IRβ (C-20, rabbit), and VEGF (P-20, goat) were from Santa Cruz Biotechnology. Chicken anti-ERβ antibody 503 was produced in this laboratory. Peroxidase-conjugated secondary antibodies, anti-rabbit IgG, and anti-goat IgG were from Sigma. FITC-conjugated anti-chicken IgG raised in the donkey was obtained from Jackson ImmunoResearch.
Histology and Immunohistochemistry.
Tissues were fixed in 4% paraformaldehyde overnight and processed routinely for paraffin embedding. Sections (4 μm) were mounted on organosilane-coated slides. Hematoxylin/eosin staining was used for histological evaluation under the light microscope. Sequential sections were used for PR, Ki-67, insulin-like growth factor 1 receptor (IGF-1Rβ), and VEGF staining. Briefly, sections were deparaffined in xylene for 30 min and then rehydrated. Continuous boiling in 10 mM citrate buffer for 15 min was done for antigen retrieval. The cooled sections were incubated in 0.5% H2O2 for 30 min to quench endogenous peroxidase and then incubated with 0.5% Triton X-100 in PBS for 5 min (20 min for Ki-67). To block the unspecific binding of secondary antibodies, sections were then incubated for 1 h at 4°C in the normal serum of the host for the secondary antibodies. Sections were then incubated with rabbit anti-PR (1:100), goat anti-Ki-67 (1:100), rabbit anti-IGF-1Rβ (1:100), or goat anti-VEGF (1:200) in 3% BSA overnight at 4°C except for the Ki-67 antibody incubation, which was performed at room temperature for 3 h. Negative controls were incubated with preadsorbed PR antibody or only 3% BSA instead of primary antibody for Ki-67, IGF-1Rβ, or VEGF. For PR, IGF-1Rβ, and VEGF, slides were washed with PBS and incubated with secondary antibody (peroxidase-conjugated goat anti-rabbit IgG) for 1 h at room temperature. After thorough washing in PBS, sections were developed with 3,3′-diaminobenzidine substrate (Dako), slightly counterstained with Mayer's hematoxylin, dehydrated and mounted with Permount. For Ki-67, the ABC method was used to visualize the signal according to the manual provided by the manufacturer (Vector Laboratories). ERβ staining was done on frozen tissue sections by using chicken anti-ERβ503 antibody as described (17).
Electrophoretic Analyses of Proteins in the Uterine Fluid.
Uterine fluids from estradiol-treated wild-type and BERKO uteri were added to SDS loading buffer, and analyzed by SDS/PAGE with 4–20% precasted gradient gel (NOVEX, San Diego). After transfer to a polyvinylidene difluoride membrane, the membrane was stained with Coomassie brilliant blue.
Reverse Transcription–PCR (RT-PCR).
Total RNA was isolated by RNAWIZ (Ambion, Austin, TX) according to the protocol provided by the company. For the reverse transcription reaction, 5 μg of RNA together with 2.2 μM oligo(dT) was denatured at 70°C for 5 min. The RNA was added to a mixture of reverse transcription reaction buffer (GIBCO/BRL), containing 30 nmol of each dNTP, 50 units of RNasin, and 20 units of SuperScript II reverse transcriptase (GIBCO/BRL) in a final volume of 30 μl. The reaction was allowed to proceed for 1 h at 46°C, after which the enzyme was inactivated at 95°C for 10 min. The reverse-transcribed RNA was stored at −20°C for further use. Two negative controls were included: one without the RNA sample and one without the reverse transcriptase.
The PCR was run on a Gene Amp PCR system 2400 (Perkin–Elmer). The reaction consisted of 3 μl of cDNA, PCR buffer, 0.2 mM dNTPs, 1 pmol of each primer, and 0.5 units of Taq polymerase in a total volume of 50 μl. Primers used are listed below together with their annealing temperatures and cycles:
ERα: forward 5′-AATTCTGACAATCGACGCCAG-3′; reverse, 5′-GTGCTTCAACATTCTCCCTCCTC-3′ (55°C, 35 cycles). ERβ: forward 5′-CTTGGTCACGTACCCCTTAC-3′; reverse 5′-GTA TCGCGTCACTTTCCTTT-3′ (55°C, 35 cycles). Complement C3: forward 5′-CCCAGTCACAGTCACTGTGCAAGA-3′; reverse 5′-ATCCGATATAAGACAGTGGAGCCA-3′ (59°C, 35 cycles). IL-1β: forward 5′-CTCCATGAGCTTTGTACACGG-3′, reverse 5′-TGCTGATGTACCAGTTGGGG-3′ (53°C, 35 cycles). IGF-1, forward 5′-CCACACTGACATGCCCAAGACTCA-3′, reverse 5′-TGTTTTGCAGGTTGCTCAAGCA-3′ (55°C, 35 cycles). β-actin, forward 5′-GGGCACAGTGTGGGTGAC-3′, reverse 5′-CTGGCACCACACCTTCTAC-3′ (53–59°C).
Results
Changes in Gross Anatomy and Morphology in the BERKO Uterus.
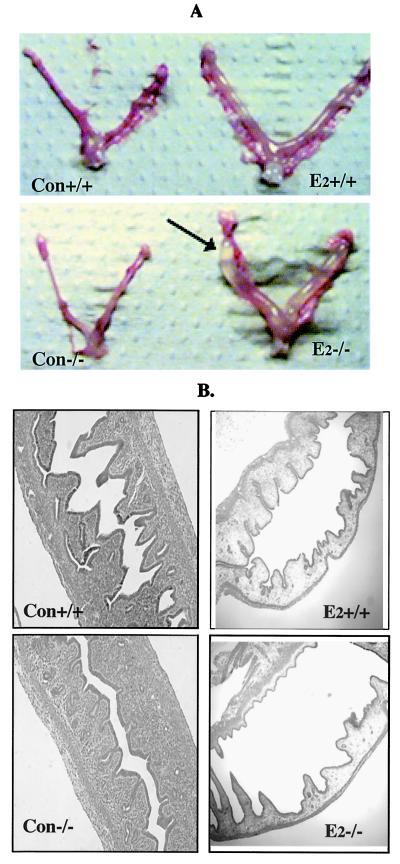
On E2 treatment, there was enlargement of the lumen in both wild-type and BERKO mice, but the lumen of the BERKO uterus became extremely large and filled with fluid (Fig. 1A).Under the light microscope, an increase in the number of glands was observed in the uteri of the untreated 21-day-old BERKO (Fig. 1B).
Figure 1.
Gross anatomic and histological comparison of uteri of immature BERKO (−/−) and wild-type (+/+) mice. (A) Gross anatomy of control (Con) and E2-treated (E2) uteri. Arrow indicates the transparent and fluid-filled E2-treated BERKO uterus compared with that of the wild type. (B) Histology of control and E2-treated uteri under the light microscope. Note the increased number of glands in the BERKO.
Changes in Uterine Secretion in the BERKO.
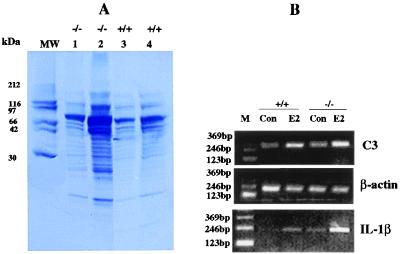
The uterine secretions harvested from the lumen of the BERKO and wild type were analyzed by SDS/PAGE (Fig. 2). Both quantitative and qualitative differences in protein secretion were found. The BERKO uterus secreted more protein than the wild type. Some proteins in the molecular weight range 10–30 kDa were clearly visible in the E2-treated BERKO uterus, but were below the level of detection in wild-type fluid as shown in Fig. 2A. One of the major proteins in uterine fluid, complement C3, was detected by RT-PCR. E2 caused a much larger increase in C3 mRNA in BERKO than it did in wild-type mice. IL-1β, the cytokine responsible for production of C3 was also induced by E2 and again the induction in BERKO was greater than that in wild-type mice. After 30 PCR cycles, the IL-1β signal could be detected in E2-treated BERKOs but not in the untreated mice or in E2-treated wild-type uterus (data not shown). After 35 cycles of PCR, it was clear that the induction of IL-1β by E2 was higher in the BERKO uterus than in wild-type mice (Fig. 2B).
Figure 2.
Protein and mRNA analysis of uterine secretion. (A) SDS/PAGE of secreted proteins from estradiol-treated BERKO (−/−) and wild-type (+/+) uteri. Lanes 1 and 3 contain the same amount of protein; lanes 2 and 4 contain the same volume of uterine fluid. (B) RT-PCR analyses of C3 and IL-1β in the uterus of immature BERKO and wild type. β-Actin was used as internal control (M, molecular size marker).
Detection and Localization of ERs in the Uterus.
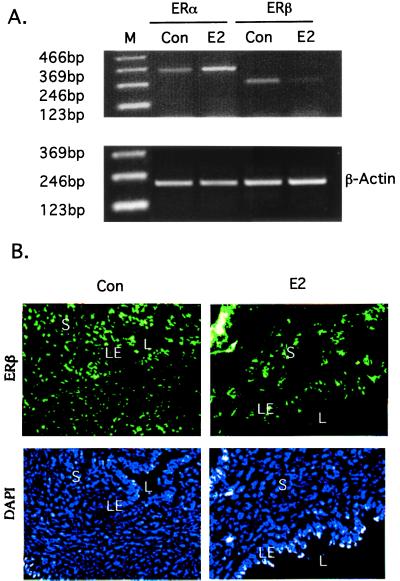
The expression of mRNA for ERα and ERβ in uteri of wild-type mice was compared by RT-PCR. In untreated animals, the levels of ERα and ERβ mRNA were similar, and E2 treatment down-regulated ERβ and up-regulated ERα (Fig. 3A). Immunofluorescent labeling revealed that ERβ protein is expressed in both epithelium and stroma. On E2 treatment, ERβ in the stroma was decreased (Fig. 3B).
Figure 3.
ERα and ERβ expression in response to E2 treatment in the wild-type uterus. (A) RT-PCR detection of ERα and ERβ mRNA. (B) Detection of ERβ by immunofluorescent labeling. (L, lumen; S, stroma; and LE, luminal epithelium). Total nuclei in the same section are indicated by fluorescence detection of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI).
Ki-67 in the BERKO.
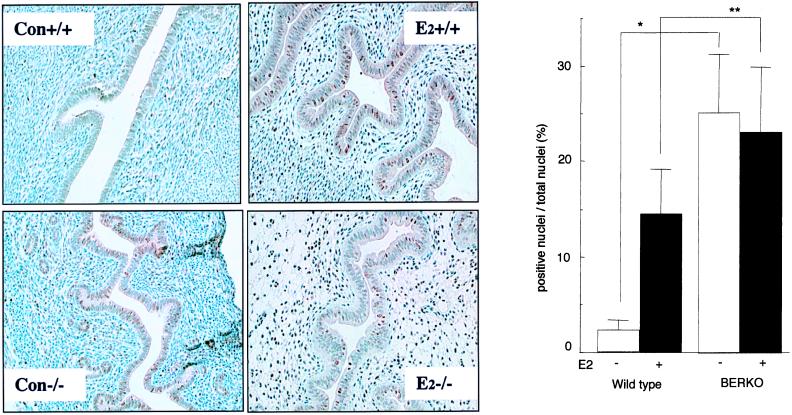
Antibodies raised against the proliferation-associated protein Ki-67 were used in immunohistochemical staining to evaluate the cell proliferation activity (Fig. 4A). Ki-67-positive nuclei were detected in 25.1 ± 6.2% of luminal epithelial cells in untreated BERKO uterus but only 2.3 ± 1.0% of epithelial cells in wild-type littermates (Fig. 4B). On E2 treatment, there was no significant change in the level of expression of Ki-67 in the BERKO uterus (23.1 ± 6.9% positive), but in the wild-type littermates, E2 elicited a sixfold (14.5 ± 4.7% positive cells) increase in Ki-67-positive nuclei in the luminal epithelium.
Figure 4.
(A) Detection of Ki-67 by immunohistochemical staining in the immature BERKO and wild-type uterus. Positive nuclear staining is brown; slides were counterstained with hematoxylin. (B) Comparison of Ki-67 positive proportions; * and ** indicate significant differences between line-linked groups (n = 3; error bar, SD; *, P < 0.01; **, P < 0.05, t-test). (Con +/+, wild-type control; Con −/−, BERKO control; E2 +/+, wild-type E2 treated; E2 −/−, BERKO E2 treated).
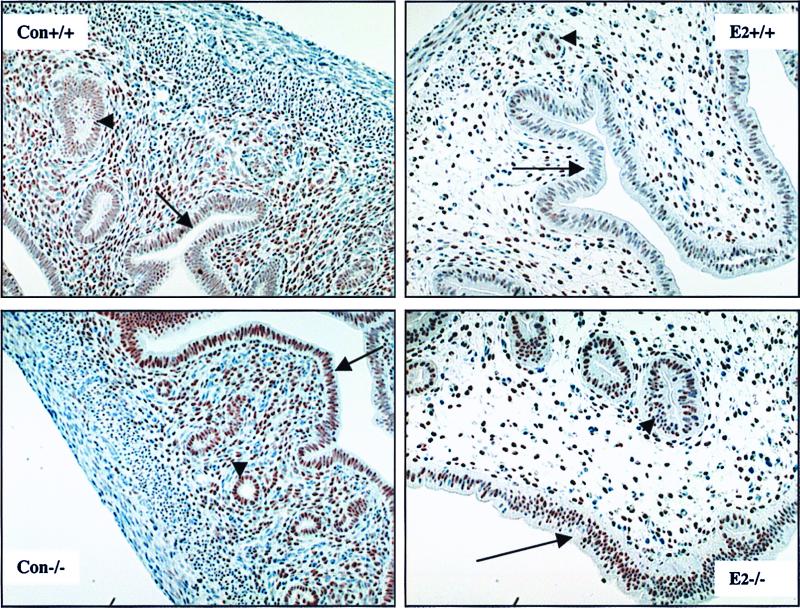
Regulation of PR.
The regulation of PR was studied by immunohistochemistry (Fig. 5). In untreated BERKOs, there is a higher expression of PR in both stroma and epithelium than in wild-type littermates. In both the wild-type and BERKO uterus, E2 induced PR in the stroma and glandular epithelial cells. In luminal epithelium, E2 decreased PR in wild-type animals but had no effect on PR expression in these cells in the BERKO.
Figure 5.
Regulation of PR by E2 in the BERKO and wild-type immature uterus. Positive nuclear staining is brown; slides were counterstained with hematoxylin. Note the higher expression of PR in the BERKO uterus in both epithelium and stroma, and that E2 treatment down-regulates PR in the luminal epithelial cells of wild-type but not BERKO uteri. (Con +/+, wild-type control; Con −/−, BERKO control; E2 +/+, wild-type E2 treated; E2 −/−, BERKO E2 treated; arrow, luminal epithelium; arrowhead, glandular epithelium).
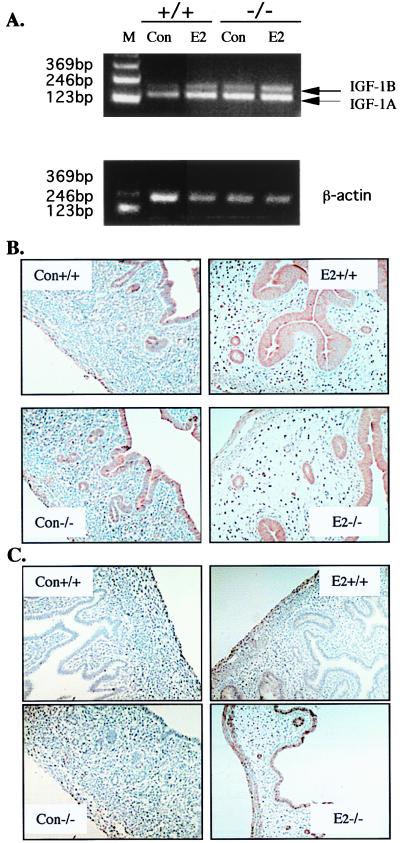
IGF-1, IGF-1R, and VEGF.
IGF-1, secreted from the stroma in response to estrogen, is a major mitogen for uterine epithelial cells. IGF-1A, IGF-1B and the receptor, IGF-1Rβ, were compared in the BERKO and wild-type littermates. IGF-1A and -1B mRNAs were assessed by RT-PCR by using one pair of primers that can discriminate between IGF-1A and -1B. It was found that IGF-1 mRNA levels for both the A and B forms were higher in untreated BERKO than in wild-type littermates. E2 treatment induced both IGF-1A and IGF-1B but IGF-1B increased more in the BERKO than in wild-type animals (Fig. 6A). IGF-1Rβ protein, on the other hand, was detected at comparable levels in the luminal epithelium of both BERKO and wild-type mice, and E2 treatment did not change IGF-1Rβ expression (Fig. 6B). Another growth factor, which is essential for uterine growth, is VEGF. The expression of VEGF was undetectable in untreated uteri and slightly induced in E2-treated wild-type uterus. In the BERKO uterus, there was an exaggerated response to E2 with VEGF expression increased in most cells in both the endometrium and myometrium (Fig. 6C).
Figure 6.
Detection of IGF-1, IGF-1Rβ, and VEGF in the BERKO and wild-type immature uterus. (A) RT-PCR analysis of IGF-1A (157 bp) and -1B (209 bp); β-actin was used as an internal control. (B) Immunohistochemical staining of IGF-1Rβ. (C) Immunohistochemical staining of VEGF. (Con +/+, wild-type control; Con −/−, BERKO control; E2 +/+, wild-type E2 treated; E2 −/−, BERKO E2 treated).
Discussion
Since its discovery in 1996 (18), several laboratories have examined the tissue distribution of ERβ in various species (19, 20). The adult uterus, which is a major estrogen target tissue, was found, in general, to have very low expression of ERβ compared with ERα (3–8, 21, 22). Furthermore, the lack of a full uterotrophic response to estrogen in female ERα knockout mice (1) suggests that ERβ plays only a minor role in the uterus. However, the reproductive performance of female BERKOs suggests otherwise. BERKO females very rarely become pregnant, and when they do, there are usually not more than one or two pups delivered. If the uteri are examined during pregnancy, there is usually implantation in only one horn, and there are usually several dead or resorbed fetuses. In this study, we have shown that, in the immature mouse uterus, ERβ and ERα are expressed at comparable levels, and most of the cells in both stroma and epithelium express ERβ. Loss of ERβ function in the immature mouse uterus causes increased expression of Ki-67, a marker of cell proliferation, and enhanced responsiveness to E2, which suggests an antiuterotrophic role of ERβ. In addition, BERKO mice lose the capacity for E2 to down-regulate PR in the luminal epithelium, indicating that induction of PR is an ERα-mediated event and repression of epithelial PR is ERβ mediated.
During postnatal development, the uterus undergoes a unique pattern of changes in cell proliferation and expression of ERα. In a systematic study (9), it was shown that the proliferation rate is high during days 1–7, when there is weak ERα expression in the stroma. The cell proliferation rate decreases sharply from day 7 until just before puberty, although during this period both the epithelium and stroma cells increase their expression of ERα. One obvious explanation for the low proliferation rate of the uterus during the prepubertal period is that estrogen levels are too low for activation of ERα (23–26). If this were the only reason for quiescence of the prepubertal uterus, the increased expression of proliferation markers in the prepubertal BERKO uterus would be difficult to understand.
One likely explanation for the BERKO phenotype is that ERβ has an antiproliferative role in the immature uterus. If this is the case, there would have to be a ligand for estrogen receptors at this stage in development. We postulate that 5α-androstane-3β,17β-diol may be such a ligand. The working hypothesis is that the ovary keeps the uterus quiescent by secreting 5α-androstane-3β,17β-diol, which activates both ERα and ERβ but ERβ represses the activity of ERα. 5α-Androstane-3β,17β-diol is produced in the ovary (27). Its circulating levels are high during the prepubertal period, but decrease sharply at the time of first ovulation (28), when the ovary starts to make enough sex hormones to initiate estrus. At puberty, the ovary secretes E2, ERβ is down-regulated, and ERα becomes fully functional. This would explain our observation that in the uterine epithelium of the untreated BERKO, about 25% of the cells are Ki-67 positive. Ki-67 is never expressed in the G0 phase of the cell cycle but is expressed during the G1, S, G2, and M phases (29). Although Ki-67 is an absolute requirement for cell proliferation, its presence does not mean that cells will divide. Cells may be arrested in any stage before M phase.
Estrogen-stimulated epithelial cell division is mediated by growth factors secreted by the stroma (11). If ERβ opposes ERα effects on epithelial cell growth, it should interfere with growth factor secretion or responsiveness. IGF-1 is the major growth factor secreted by the uterine stroma in response to E2 (30). In the BERKO uterus, there is increased expression of IGF-1 mRNA, but the expression of IGF-1 receptor β protein in the luminal epithelium is not changed. The induction of IGF-1 in the BERKO uterus suggests that induction of IGF-1 is ERα dependent. The enhanced expression of IGF-1 in the BERKO uterus and the down-regulation of ERβ in the stroma but not epithelium of the wild-type uterus in response to E2 suggests that ERβ plays an inhibitory role in the expression of IGF-1 in the uterus. VEGF, another growth factor in the uterus, is positively correlated with vessel permeability (31). The exaggerated induction of VEGF by E2 in the BERKO uterus again suggests an antiuterotrophic role of ERβ. The enhanced expression of IGF-1 and VEGF would explain the increased epithelial cell proliferation rate and exaggerated secretion in the BERKO uterus.
It is still too early to conclude that the poor reproductive performance in adult BERKO females is related to the abnormalities in the immature BERKO uterus described in this study. However, cell replication at inappropriate times in development usually has consequences for the maturation and physiological functions in mature tissue. These could result from depletion of stem cell populations, increased mutation rates, and changes in the ratio of various cell types in the tissue.
Acknowledgments
We thank AnneMarie Witte for genotyping. This study was supported by grants from KaroBio AB, the Swedish Cancer Fund, and the Deutsche Krebshilfe. S.S. has a fellowship from the Wenner-Gren Foundation.
Abbreviations
- ER
estrogen receptor
- BERKO
ERβ knockout
- E2
17β-estradiol
- PR
progesterone receptor
- IGF-1
insulin-like growth factor 1
- IGF-1R
insulin-like growth factor 1 receptor
- VEGF
vascular endothelial growth factor
- RT-PCR
reverse transcription–PCR
References
- 1.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orimo A, Inoue S, Minowa O, Tominaga N, Tomioka Y, Sato M, Kuno J, Hiro H, Shimizu Y, Suzuki M, et al. Proc Natl Acad Sci USA. 1999;96:12027–12032. doi: 10.1073/pnas.96.21.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Mol Hum Reprod. 1999;5:559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- 4.Wu J J, Geimonen E, Andersen J. Eur J Endocrinol. 2000;142:92–99. doi: 10.1530/eje.0.1420092. [DOI] [PubMed] [Google Scholar]
- 5.Sauders P T K, Maguire S M, Gaughan J, Miller M R. J Endocrinol. 1997;154:R13–R16. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- 6.Pelletier G, Luu-The V, Charbonneau A, Labrie F. Biol Reprod. 1999;61:1249–1255. doi: 10.1095/biolreprod61.5.1249. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto J, Hirose R, Sakaguchi H, Tamaya T. Mol Hum Reprod. 1999;5:742–747. doi: 10.1093/molehr/5.8.742. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Masironi B, Eriksson H, Sahlin L. Biol Reprod. 1999;61:955–964. doi: 10.1095/biolreprod61.4.955. [DOI] [PubMed] [Google Scholar]
- 9.Li S. Histochemistry. 1994;102:405–413. doi: 10.1007/BF00268912. [DOI] [PubMed] [Google Scholar]
- 10.Martin L, Finn C A, Trinder G. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 11.Cooke P S, Buchanan D L, Young P, Setiawan T, Brody J, Korach K S, Taylor J, Lubahn D B, Cunha G R. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibbetts T A, Mendoza-Meneses M, O'Malley B W, Conneely O M. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 13.Paech K, Webb P, Nisson S, Gustafsson J-Å, Kushner P J, Scanlan T S. Science. 1997;177:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 14.Hall J M, McDonnell D P. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 15.Pettersson K, Grandien K, Kuiper G G J M, Gustafsson J-Å. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 16.Cowley S M, Hoare S, Mosselman S, Parker M G. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 17.Saji S, Jensen E V, Nilsson S, Rylander T, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson S, Kuiper G G J M, Gustafsson J-Å. Trends Endocrinol Metab. 1998;9:387–395. doi: 10.1016/s1043-2760(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 20.Warner M, Nilsson S, Gustafsson J-Å. Curr Opin Obstet Gynecol. 1999;11:249–254. doi: 10.1097/00001703-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper G G J M, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-Å. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Couse J F, Lindzey J, Grandien K, Gustafsson J-Å, Korach K S. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 23.Meijs-Roelofs H M, Uilenbroek J T, De Greef W J, De Jong F H, Kramer P. J Endocrinol. 1975;67:275–282. doi: 10.1677/joe.0.0670275. [DOI] [PubMed] [Google Scholar]
- 24.Parker C R J, Mahesh V B. Biol Reprod. 1976;14:347–353. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- 25.Advis J P, Andrews W W, Ojeda S R. Endocrinology. 1979;104:652–658. doi: 10.1210/endo-104-3-653. [DOI] [PubMed] [Google Scholar]
- 26.Andrews W W, Mizejewski G J, Ojeda S R. Endocrinology. 1981;109:1404–1413. doi: 10.1210/endo-109-5-1404. [DOI] [PubMed] [Google Scholar]
- 27.Eckstein B. J Steroid Biochem. 1983;19:883–886. doi: 10.1016/0022-4731(83)90028-6. [DOI] [PubMed] [Google Scholar]
- 28.Ruf K B. J Steroid Biochem. 1983;19:887–890. doi: 10.1016/0022-4731(83)90029-8. [DOI] [PubMed] [Google Scholar]
- 29.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 30.Rutanen E-M. Gynecol Endocrinol. 1998;12:399–406. doi: 10.3109/09513599809012842. [DOI] [PubMed] [Google Scholar]
- 31.Cullinan-Bove K, Koos R D. Endocrinology. 1993;133:829–837. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]