Abstract
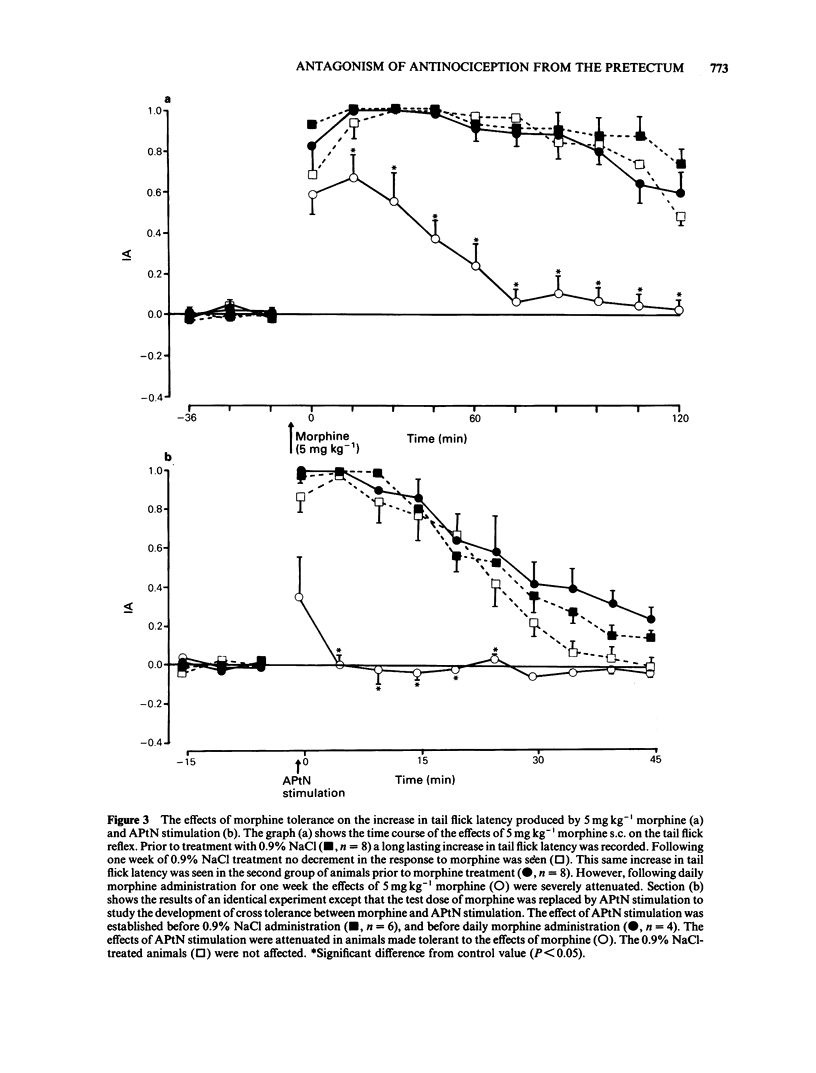
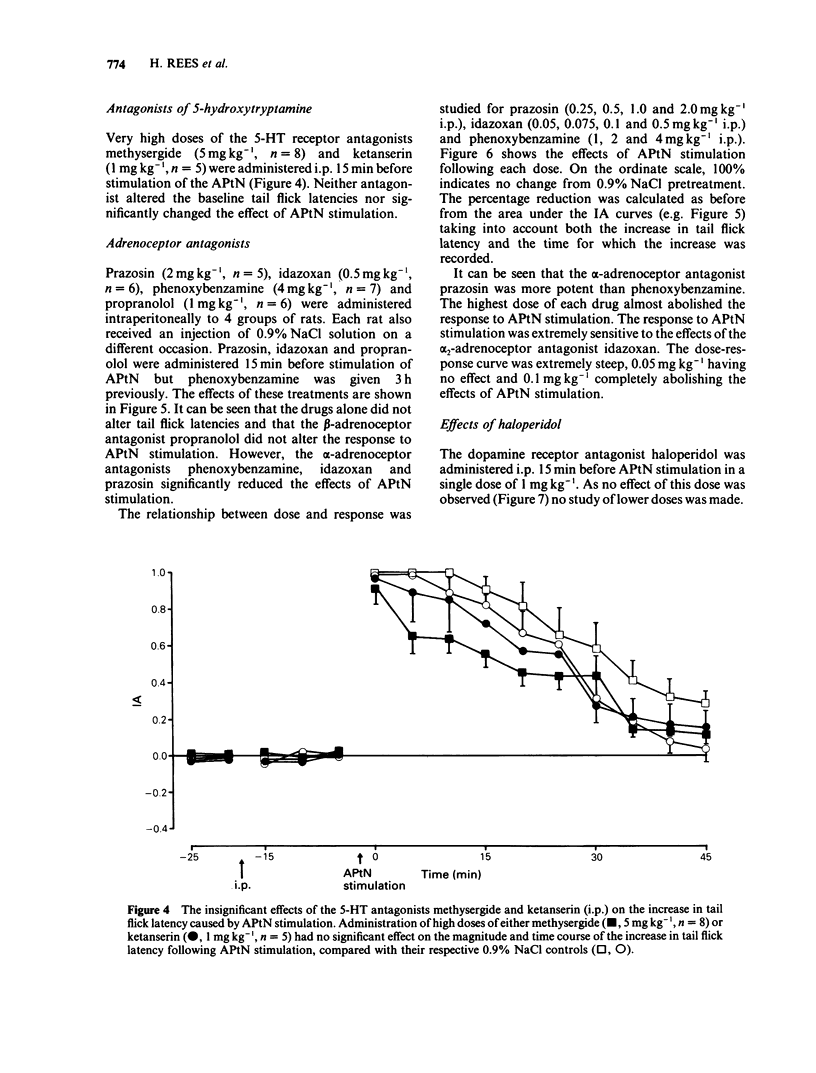
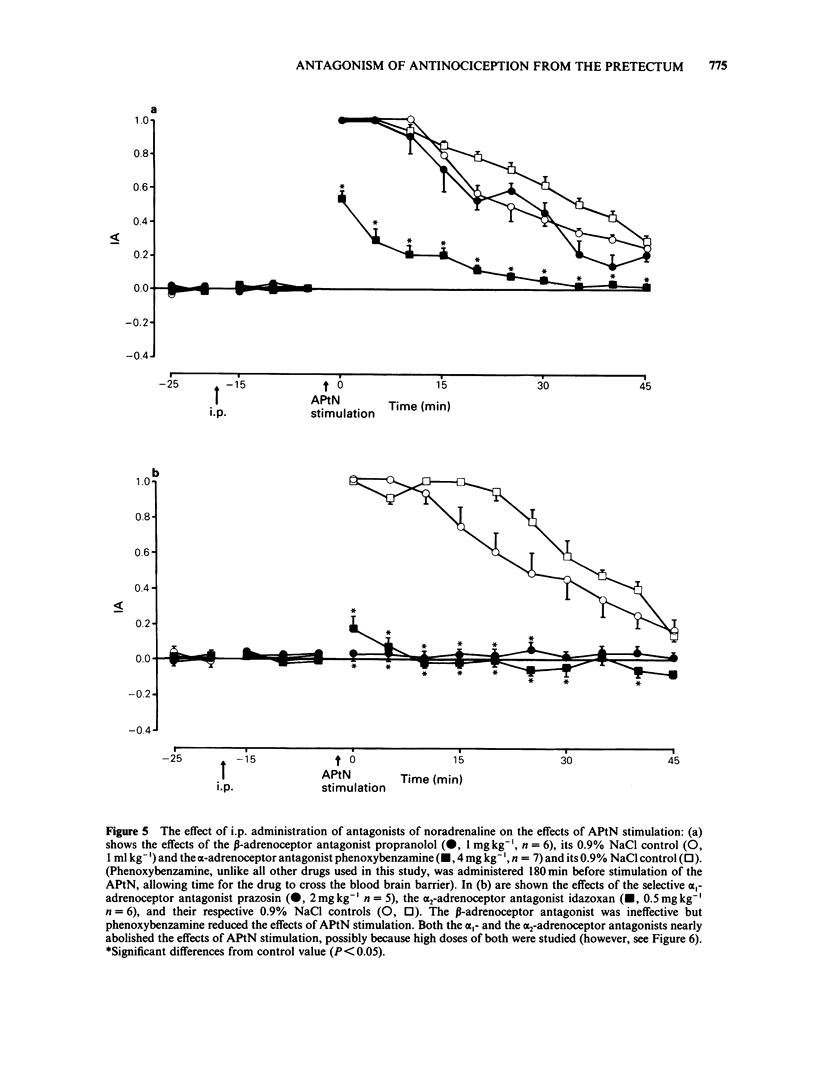
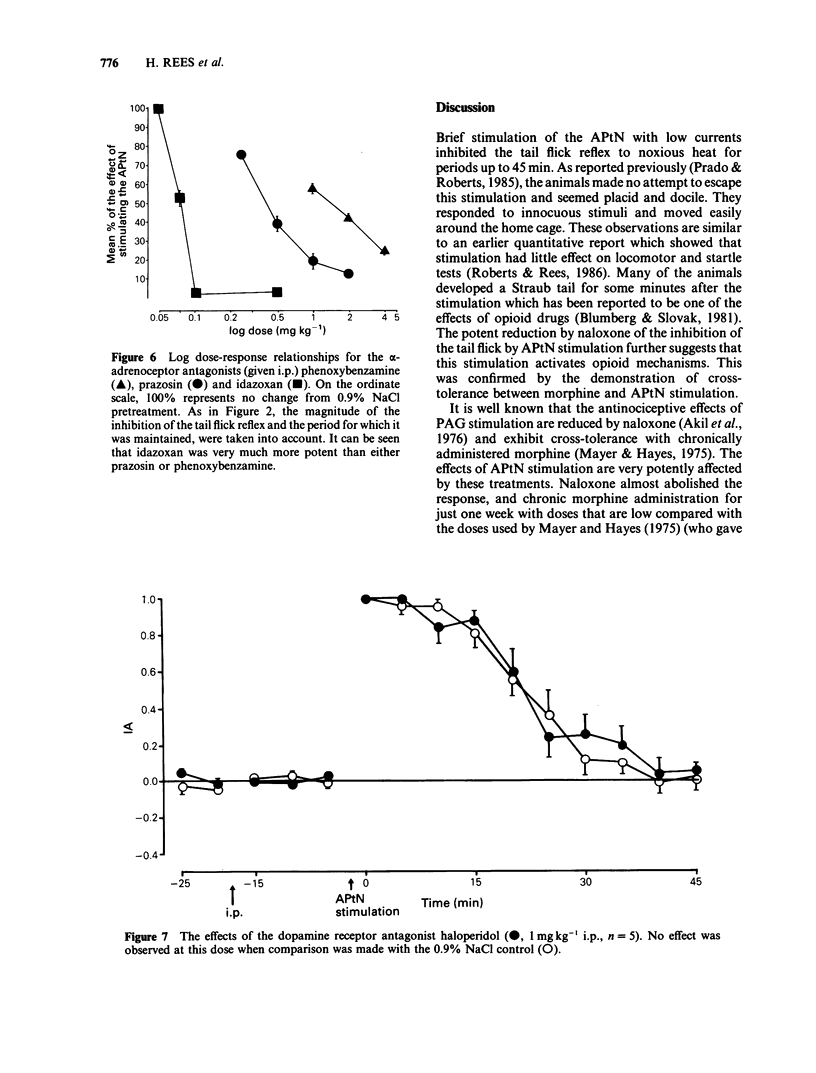
1 The effects of intraperitoneal administration of antagonists to morphine, 5-hydroxytryptamine (5-HT), noradrenaline and dopamine have been studied on the antinociceptive effects of electrical stimulation of the anterior pretectal nucleus (APtN) of the rat. 2 A 15 s period of 35 microA sine wave stimulation of APtN significantly increased the latency of the tail flick reflex to noxious heat for periods up to 1 h. 3 Naloxone (0.25-1.0 mg kg-1) attenuated the effects of APtN stimulation in a dose-dependent manner. In rats made tolerant to morphine by daily administration of morphine, the antinociceptive effects of APtN stimulation were significantly reduced. 4 The 5-HT receptor antagonists methysergide (5 mg kg-1) and ketanserin (1 mg kg-1), the dopamine receptor antagonist haloperidol (1 mg kg-1) and the beta-adrenoceptor antagonist propranolol (1 mg kg-1) had little effect on the antinociceptive effects of stimulating the APtN. 5 alpha-Adrenoceptor antagonists caused a dose-dependent antagonism of the response. The order of potency was; idazoxan greater than prazosin greater than phenoxybenzamine, the respective ED50 for each drug being 0.08: 0.45: 1.5 mg kg-1. 6 It is concluded that antagonism at opioid receptors and alpha-adrenoceptors but not beta-adrenoceptors, dopamine or 5-HT receptors reduces the antinociceptive effects of APtN stimulation. This differs from the reported effects of these antagonists on the antinociception caused by stimulating other sites in the brain.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Mayer D. J., Liebeskind J. C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Azami J., Llewelyn M. B., Roberts M. H. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982 Mar;12(3):229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Barbaro N. M., Hammond D. L., Fields H. L. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985 Sep 23;343(2):223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978 Nov;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979 Oct 1;187(3):513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W., Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Res. 1978 Aug 4;151(2):307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Berkley K. J., Mash D. C. Somatic sensory projections to the pretectum in the cat. Brain Res. 1978 Dec 15;158(2):445–449. doi: 10.1016/0006-8993(78)90687-x. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Slovak D. M. Effects of yohimbine and naltrexone in counteraction of the morphine Straub tail and the amphetamine-potentiated morphine Straub tail in mice. Life Sci. 1982 Jun 14;30(24):2115–2121. doi: 10.1016/0024-3205(82)90454-4. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Camarata P. J., Yaksh T. L. Characterization of the spinal adrenergic receptors mediating the spinal effects produced by the microinjection of morphine into the periaqueductal gray. Brain Res. 1985 Jun 10;336(1):133–142. doi: 10.1016/0006-8993(85)90424-x. [DOI] [PubMed] [Google Scholar]
- Carstens E., Fraunhoffer M., Zimmermann M. Serotonergic mediation of descending inhibition from midbrain periaqueductal gray, but not reticular formation, or spinal nociceptive transmission in the cat. Pain. 1981 Apr;10(2):149–167. doi: 10.1016/0304-3959(81)90191-3. [DOI] [PubMed] [Google Scholar]
- Commissiong J. W., Hellström S. O., Neff N. H. A new projection from locus coeruleus to the spinal ventral columns: histochemical and biochemical evidence. Brain Res. 1978 Jun 9;148(1):207–213. doi: 10.1016/0006-8993(78)90391-8. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker S. M., Mitchell R., Hope P. J., Molony V., Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 1985 May 20;334(2):243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Griersmith B. T., Duggan A. W., North R. A. Methysergide and supraspinal inhibition of the spinal transmission of nociceptive information in the anaesthetized cat. Brain Res. 1981 Jan 5;204(1):147–158. doi: 10.1016/0006-8993(81)90658-2. [DOI] [PubMed] [Google Scholar]
- Guilbaud G., Besson J. M., Oliveras J. L., Liebeskind J. C. Suppression by LSD of the inhibitory effect exerted by dorsal raphe stimulation on certain spinal cord interneurons in the cat. Brain Res. 1973 Oct 26;61:417–422. doi: 10.1016/0006-8993(73)90549-0. [DOI] [PubMed] [Google Scholar]
- Hayes R. L., Newlon P. G., Rosecrans J. A., Mayer D. J. Reduction of stimulation-produced analgesia by lysergic acid deithylamide, a depressor of serotonergic neural activity. Brain Res. 1977 Feb 18;122(2):367–372. doi: 10.1016/0006-8993(77)90304-3. [DOI] [PubMed] [Google Scholar]
- Hayes R., Price D. D., Dubner R. Naloxone antagonism as evidence for narcotic mechanisms. Science. 1977 May 6;196(4290):600–600. doi: 10.1126/science.196.4290.600. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y., Adams J. E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977 Jul 8;197(4299):183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y., Harada Y., Takagi H. Noradrenaline regulation of pain-transmission in the spinal cord mediated by alpha-adrenoceptors. Brain Res. 1979 Oct 5;174(2):333–336. doi: 10.1016/0006-8993(79)90857-6. [DOI] [PubMed] [Google Scholar]
- Kuypers H. G., Maisky V. A. Funicular trajectories of descending brain stem pathways in cat. Brain Res. 1977 Nov 4;136(1):159–165. doi: 10.1016/0006-8993(77)90141-x. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- Llewelyn M. B., Azami J., Roberts M. H. The effect of modification of 5-hydroxytryptamine function in nucleus raphe magnus on nociceptive threshold. Brain Res. 1984 Jul 23;306(1-2):165–170. doi: 10.1016/0006-8993(84)90365-2. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Hayes R. L. Stimulation-produced analgesia: development of tolerance and cross-tolerance to morphine. Science. 1975 May 30;188(4191):941–943. doi: 10.1126/science.1094537. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Price D. D. Central nervous system mechanisms of analgesia. Pain. 1976 Dec;2(4):379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Monroe P. J., Smith D. J. Characterization of multiple [3H]5-hydroxytryptamine binding sites in rat spinal cord tissue. J Neurochem. 1983 Aug;41(2):349–355. doi: 10.1111/j.1471-4159.1983.tb04749.x. [DOI] [PubMed] [Google Scholar]
- Nygren L. G., Olson L. A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res. 1977 Aug 19;132(1):85–93. doi: 10.1016/0006-8993(77)90707-7. [DOI] [PubMed] [Google Scholar]
- Prado W. A., Roberts M. H. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res. 1985 Aug 12;340(2):219–228. doi: 10.1016/0006-8993(85)90917-5. [DOI] [PubMed] [Google Scholar]
- Reddy S. V., Yaksh T. L. Spinal noradrenergic terminal system mediates antinociception. Brain Res. 1980 May 12;189(2):391–401. doi: 10.1016/0006-8993(80)90099-2. [DOI] [PubMed] [Google Scholar]
- Rees H., Roberts M. H. Anterior pretectal stimulation alters the responses of spinal dorsal horn neurones to cutaneous stimulation in the rat. J Physiol. 1987 Apr;385:415–436. doi: 10.1113/jphysiol.1987.sp016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Rivot J. P., Chaouch A., Besson J. M. Nucleus raphe magnus modulation of response of rat dorsal horn neurons to unmyelinated fiber inputs: partial involvement of serotonergic pathways. J Neurophysiol. 1980 Dec;44(6):1039–1057. doi: 10.1152/jn.1980.44.6.1039. [DOI] [PubMed] [Google Scholar]
- Roberts M. H. 5-Hydroxytryptamine and antinociception. Neuropharmacology. 1984 Dec;23(12B):1529–1536. doi: 10.1016/0028-3908(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Rees H. The antinociceptive effects of stimulating the pretectal nucleus of the rat. Pain. 1986 Apr;25(1):83–93. doi: 10.1016/0304-3959(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Hammond D. L., Ochi J. W., Yaksh T. L. Pharmacological antagonism of the antinociceptive effects of serotonin in the rat spinal cord. Eur J Pharmacol. 1983 Jun 17;90(4):349–357. doi: 10.1016/0014-2999(83)90556-3. [DOI] [PubMed] [Google Scholar]
- Tohyama M., Sakai K., Salvert D., Touret M., Jouvet M. Spinal projections from the lower brain stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectories. Brain Res. 1979 Sep 21;173(3):383–403. doi: 10.1016/0006-8993(79)90237-3. [DOI] [PubMed] [Google Scholar]


