Abstract
1 The effects of quinine sulphate, tetramethylammonium chloride (TMA) and tetraethylammonium chloride (TEA) (all blockers of the Ca2+-activated K+ channels) on the relaxations induced by acetylcholine (ACh), calcium ionophore A23187 and sodium nitrite were studied in helical strips of rabbit aorta. 2 The strips were contracted to a moderate stable tone with phenylephrine (10(-7) M). ACh (4 X 10(-9) to 10(-6) M) as well as A23187 (10(-8) to 3 X 10(-7) M) reduced this tone in a concentration- and endothelium-dependent manner. 3 Pretreatment of the tissues with quinine (2.5 X 10(-5) to 10(-4) M) for 60 min produced a concentration-dependent inhibition of the relaxation induced by ACh. Also 90 min incubation of the strips with TMA (3 X 10(-3) to 6.5 X 10(-2) M) or TEA (10(-3) to 3 X 10(-2) M) inhibited the ACh-evoked relaxation in a manner similar to quinine. 4 Quinine (10(-4) M, 60 min), TMA (6.5 X 10(-2) M, 90 min) or TEA (3 X 10(-2) M, 90 min) produced 5 to 10 fold reductions in the relaxant EC50 values of A23187 and ACh and depressed (by 40 to 95%) the maximal relaxations to the ionophore and ACh. 5 On a molar basis, quinine was more effective than the two tetraalkylammonium ions in reducing the endothelium-dependent relaxations of the aortic strips induced by ACh or A23187. The inhibitory actions were reversible after 60 to 90 min washout.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

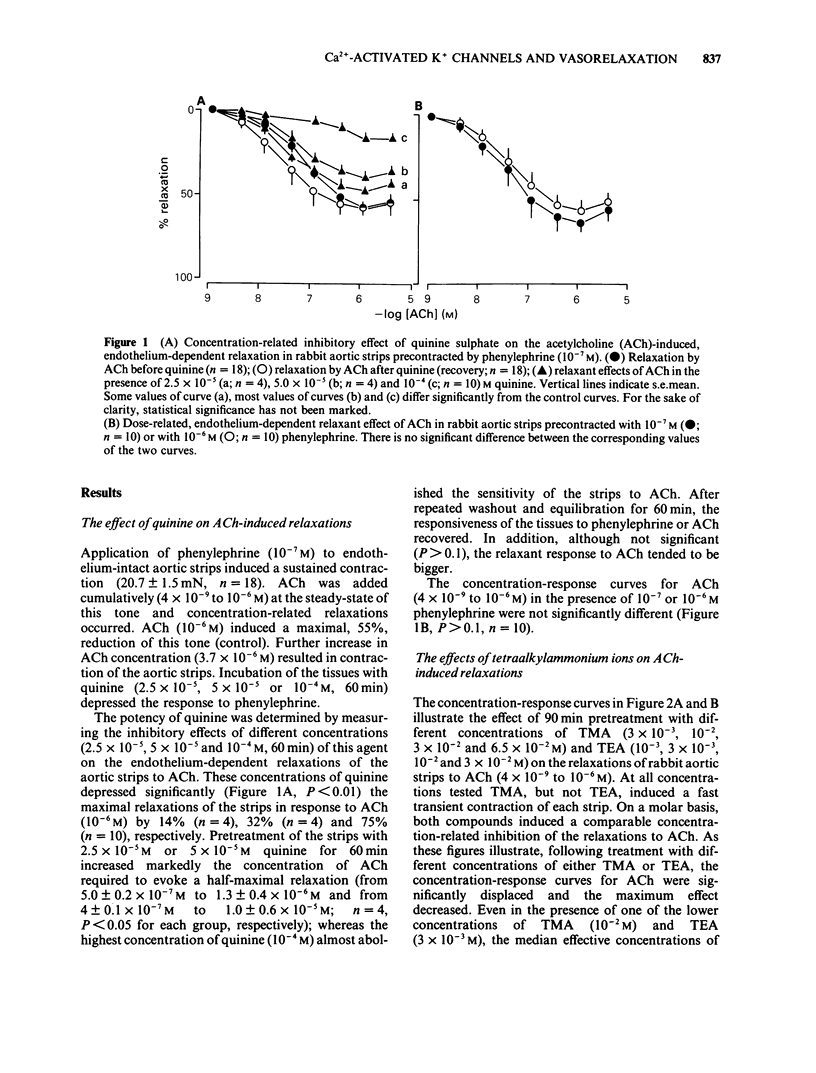
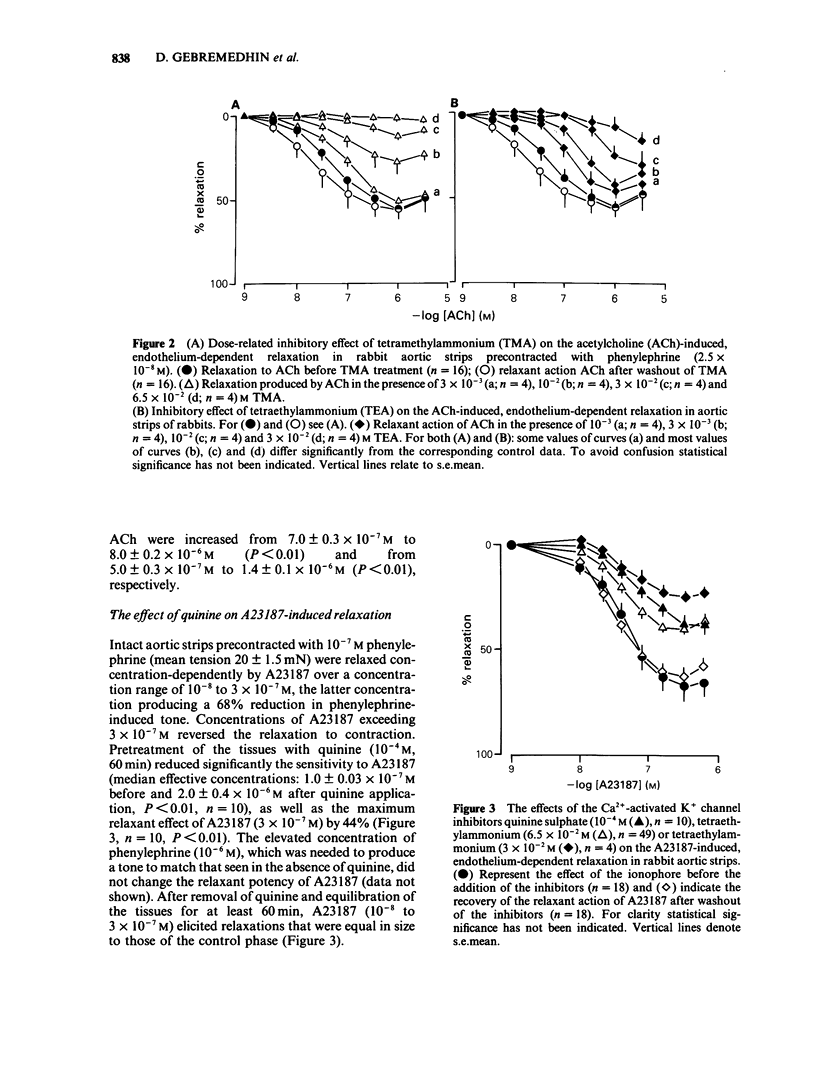
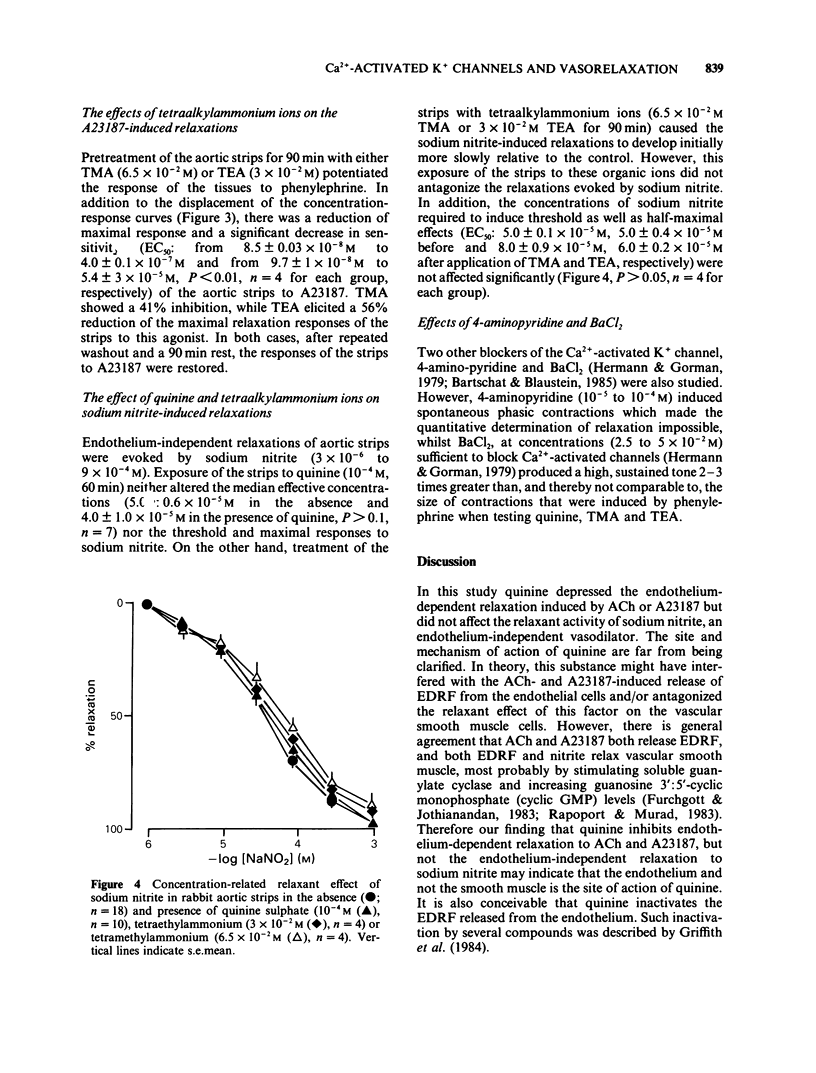


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armando-Hardy M., Ellory J. C., Ferreira H. G., Fleminger S., Lew V. L. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol. 1975 Aug;250(1):32P–33P. [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Bartschat D. K., Blaustein M. P. Calcium-activated potassium channels in isolated presynaptic nerve terminals from rat brain. J Physiol. 1985 Apr;361:441–457. doi: 10.1113/jphysiol.1985.sp015654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Couturier E., Sener A., Anjaneyulu K., Malaisse W. J. Inhibition by quinine of insulin release and calcium ionophoresis. Mol Pharmacol. 1980 Sep;18(2):243–246. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Interaction between Na+,K+ exchanges and the direct inhibitory effect of acetylcholine on canine femoral arteries. Circ Res. 1980 Jun;46(6):826–836. doi: 10.1161/01.res.46.6.826. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F., BHADRAKOM S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953 Jun;108(2):129–143. [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P., De la Lande I. S., Jellett L. B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther. 1972 May;181(2):339–345. [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. An ion's view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J Gen Physiol. 1985 May;85(5):669–698. doi: 10.1085/jgp.85.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C. Effect of tetraethylammonium on noradrenaline release from cat spleen treated with tetrodotoxin. Nature. 1978 Dec 7;276(5688):623–624. doi: 10.1038/276623a0. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Mecca T. E., Elam J. T., Nash C. B., Caldwell R. W. alpha-Adrenergic blocking properties of quinine HCl. Eur J Pharmacol. 1980 May 2;63(2-3):159–166. doi: 10.1016/0014-2999(80)90439-2. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985 Apr 2;110(2):203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Ca++-activated K+ channels in vertebrate smooth muscle cells. Cell Calcium. 1983 Dec;4(5-6):321–330. doi: 10.1016/0143-4160(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15(1-3):198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]


