Abstract
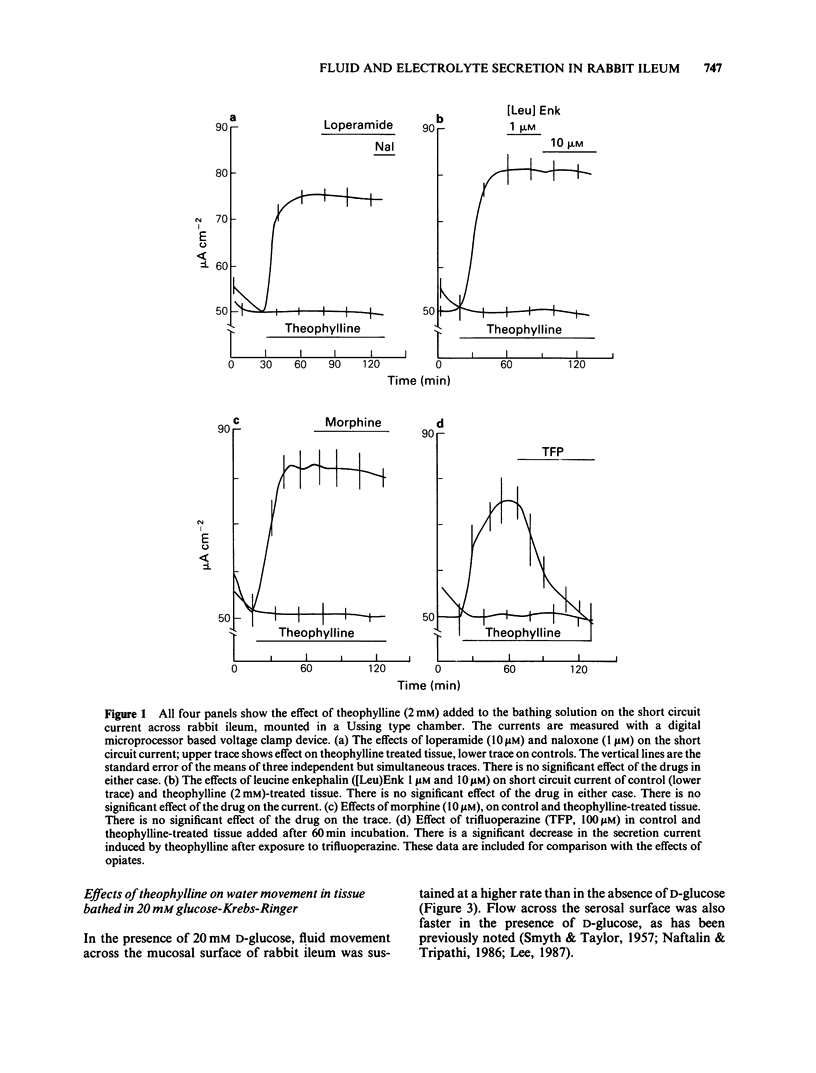
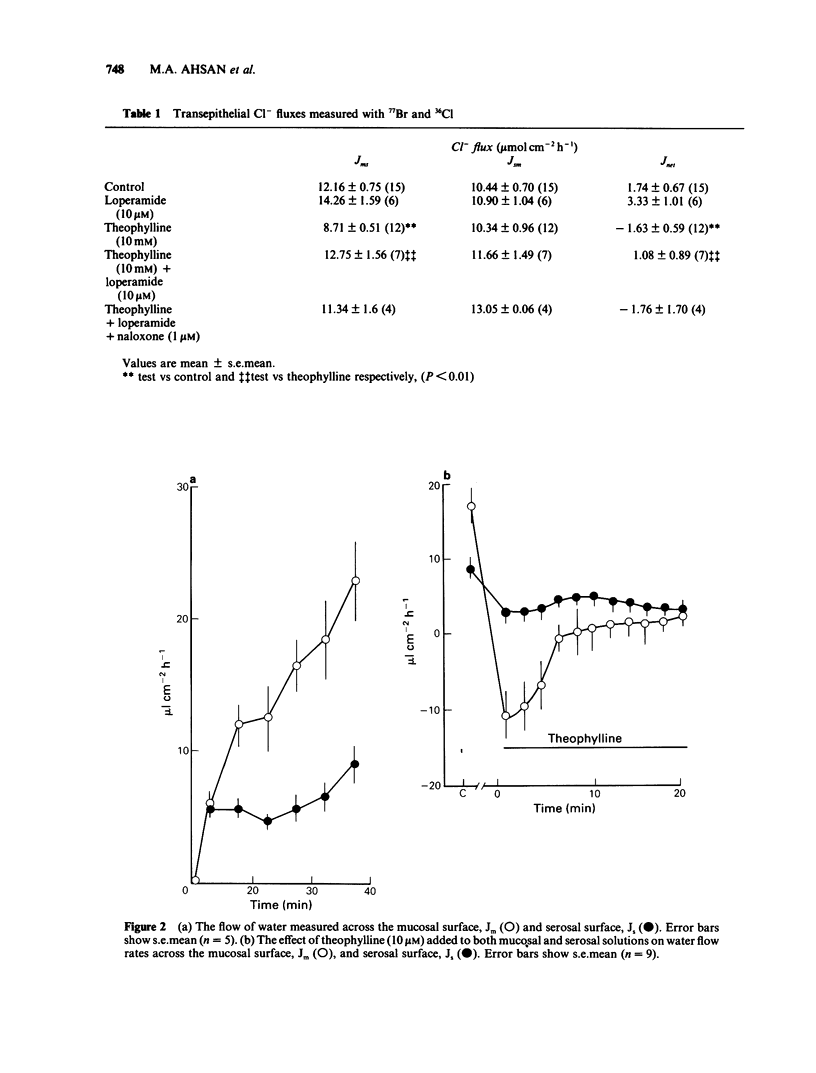
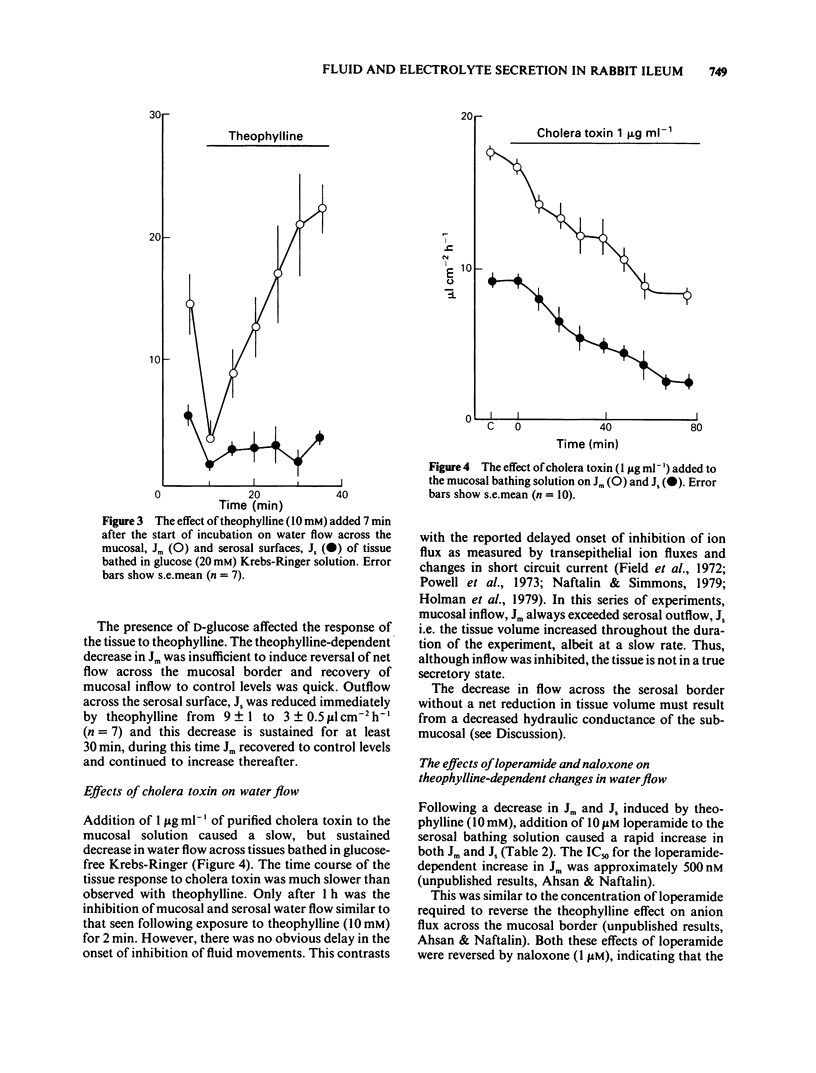
1 The effects of theophylline and cholera toxin on water and anion movements across rabbit ileum in vitro and the reversal of these effects by the opiate action of loperamide have been investigated. Water movement across the mucosal and serosal surfaces of the tissue was measured continuously by a high resolution method. 2 Theophylline caused an increase in short circuit current and reversed the direction of net C1- movement, due mainly to a decrease in mucosal-serosal flux. It also caused a rapid, but transient, reversal in the direction of fluid movement across the mucosal surface. Fluid outflow across the serosal surface was decreased but not reversed. Cholera toxin caused a slow inhibition of water movement across both mucosal and serosal surfaces. 3 Theophylline increased the exit rate of 77Br across the mucosal surface and decreased the exit rate of 77Br across the serosal surface. Theophylline increased the exit rate of 3H-labelled mannitol across the mucosal surface. 4 Loperamide reversed the effects of theophylline and cholera toxin on water flow across the mucosal and serosal surfaces and on net transepithelial C1- flux; it also increased the rate of 77Br exit across the serosal surface of theophylline-treated tissue. These effects of loperamide could be reversed by naloxone. 5 The hydraulic conductivity, Lp of the serosal surface was measured directly by determining the osmotic flow generated by low concentrations of polyethylene glycol (mol. wt. 20,000 and 90,000). Theophylline reduced the Lp by 57%. Loperamide added to theophylline-treated tissues increased the Lp by 340%. This effect was reversed by naloxone. 6 These results indicate that modulation of intestinal smooth muscle tone affects transepithelial ion and water flows in vitro. The increase in tone induced by secretagogues increases ion and water reflux via wide shunt channels in the mucosa and thereby reduces net absorption. The increased net fluid and electrolyte absorption induced by loperamide results from the opiate-dependent inhibition of acetylcholine release from intrinsic ganglia which reduces smooth muscle tone and thereby enhances the fluid and electrolyte conductance of the submucosal layers.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker G. F., Segal M. B. The effect of loperamide on the ion fluxes across the isolated rabbit colon. Biochem Pharmacol. 1981 Dec 15;30(24):3371–3373. doi: 10.1016/0006-2952(81)90616-x. [DOI] [PubMed] [Google Scholar]
- Beubler E., Lembeck F. Inhibition by morphine of prostaglandin E1-stimulated secretion and cyclic adenosine 3',5'-monophosphate formation in the rat jejunum in vivo. Br J Pharmacol. 1980 Mar;68(3):513–518. doi: 10.1111/j.1476-5381.1980.tb14566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Aceves J., Mandel L. J. Potassium uptake across serosal surface of isolated frog skin epithelium. Am J Physiol. 1972 Jun;222(6):1366–1373. doi: 10.1152/ajplegacy.1972.222.6.1366. [DOI] [PubMed] [Google Scholar]
- Cooke H. J. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol. 1984 Mar;246(3 Pt 1):G263–G267. doi: 10.1152/ajpgi.1984.246.3.G263. [DOI] [PubMed] [Google Scholar]
- Coupar I. M. Inhibition by morphine of prostaglandin-stimulated fluid secretion in rat jejunum. Br J Pharmacol. 1978 May;63(1):57–63. doi: 10.1111/j.1476-5381.1978.tb07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A. L., Kosterlitz H. W., Waterfield A. A. Factors influencing the release of acetylcholine from the myenteric plexus of the ileum of the guinea-pig and rabbit. Br J Pharmacol. 1978 Dec;64(4):565–580. doi: 10.1111/j.1476-5381.1978.tb17319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins J., Racusen L., Binder H. J. Effect of D-alanine methionine enkephalin amide on ion transport in rabbit ileum. J Clin Invest. 1980 Jul;66(1):19–28. doi: 10.1172/JCI109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farack U. M., Kautz U., Loeschke K. Loperamide reduces the intestinal secretion but not the mucosal cAMP accumulation induced by choleratoxin. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):178–179. doi: 10.1007/BF00500077. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Schulzke J. D., Hegel U. Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch. 1985 Dec;405(4):400–402. doi: 10.1007/BF00595695. [DOI] [PubMed] [Google Scholar]
- Gaginella T. S., Rimele T. J., Wietecha M. Studies on rat intestinal epithelial cell receptors for serotonin and opiates. J Physiol. 1983 Feb;335:101–111. doi: 10.1113/jphysiol.1983.sp014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Shackleford J. S., Taylor A. E. PGE1-induced intestinal secretion: mechanism of enhanced transmucosal protein efflux. Am J Physiol. 1979 Jun;236(6):E788–E796. doi: 10.1152/ajpendo.1979.236.6.E788. [DOI] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Read N. W., Redfern J. S. The action of loperamide in inhibiting prostaglandin-induced intestinal secretion in the rat. Br J Pharmacol. 1981 Nov;74(3):563–569. doi: 10.1111/j.1476-5381.1981.tb10465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal ion transport: effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976 Jul;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S. Relationship between intestinal motility, tone, water absorption and lymph flow in the rat. J Physiol. 1983 Dec;345:489–499. doi: 10.1113/jphysiol.1983.sp014991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay J. S., Linaker B. D., Turnberg L. A. Influence of opiates on ion transport across rabbit ileal mucosa. Gastroenterology. 1981 Feb;80(2):279–284. [PubMed] [Google Scholar]
- Merritt J. E., Brown B. L., Tomlinson S. Loperamide and calmodulin. Lancet. 1982 Jan 30;1(8266):283–283. doi: 10.1016/s0140-6736(82)91006-6. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Holman G. D. The role of Na as a determinant of the asymmetric permeability of rabbit ileal brush-border to D-galactose. Biochim Biophys Acta. 1974 Dec 24;373(3):453–470. doi: 10.1016/0005-2736(74)90025-x. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Simmons N. L. The effects of theophylline and choleragen on sodium and chloride ion movements within isolated rabbit ileum. J Physiol. 1979 May;290(2):331–350. doi: 10.1113/jphysiol.1979.sp012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J Physiol. 1985 Mar;360:27–50. doi: 10.1113/jphysiol.1985.sp015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Tripathi S. The roles of paracellular and transcellular pathways and submucosal space in isotonic water absorption by rabbit ileum. J Physiol. 1986 Jan;370:409–432. doi: 10.1113/jphysiol.1986.sp015942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Coupled sodium-chloride influx across the brush border of rabbit ileum. Am J Physiol. 1973 Aug;225(2):467–475. doi: 10.1152/ajplegacy.1973.225.2.467. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. U., Reuss L. Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1983 May;81(5):705–729. doi: 10.1085/jgp.81.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Binder H. J., Curran P. F. Active electrolyte secretion stimulated by choleragen in rabbit ileum in vitro. Am J Physiol. 1973 Oct;225(4):781–787. doi: 10.1152/ajplegacy.1973.225.4.781. [DOI] [PubMed] [Google Scholar]
- SMYTH D. H., TAYLOR C. B. Transfer of water and solutes by an in vitro intestinal preparation. J Physiol. 1957 May 23;136(3):632–648. doi: 10.1113/jphysiol.1957.sp005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu B. K., Tripp J. H., Candy D. C., Harries J. T. Loperamide: studies on its mechanism of action. Gut. 1981 Aug;22(8):658–662. doi: 10.1136/gut.22.8.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjövall H., Brunsson I., Jodal M., Lundgren O. The effect of vagal nerve stimulation on net fluid transport in the small intestine of the cat. Acta Physiol Scand. 1983 Mar;117(3):351–357. doi: 10.1111/j.1748-1716.1983.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Zavecz J. H., Jackson T. E., Limp G. L., Yellin T. O. Relationship between anti-diarrheal activity and binding to calmodulin. Eur J Pharmacol. 1982 Mar 12;78(3):375–377. doi: 10.1016/0014-2999(82)90042-5. [DOI] [PubMed] [Google Scholar]


