Abstract
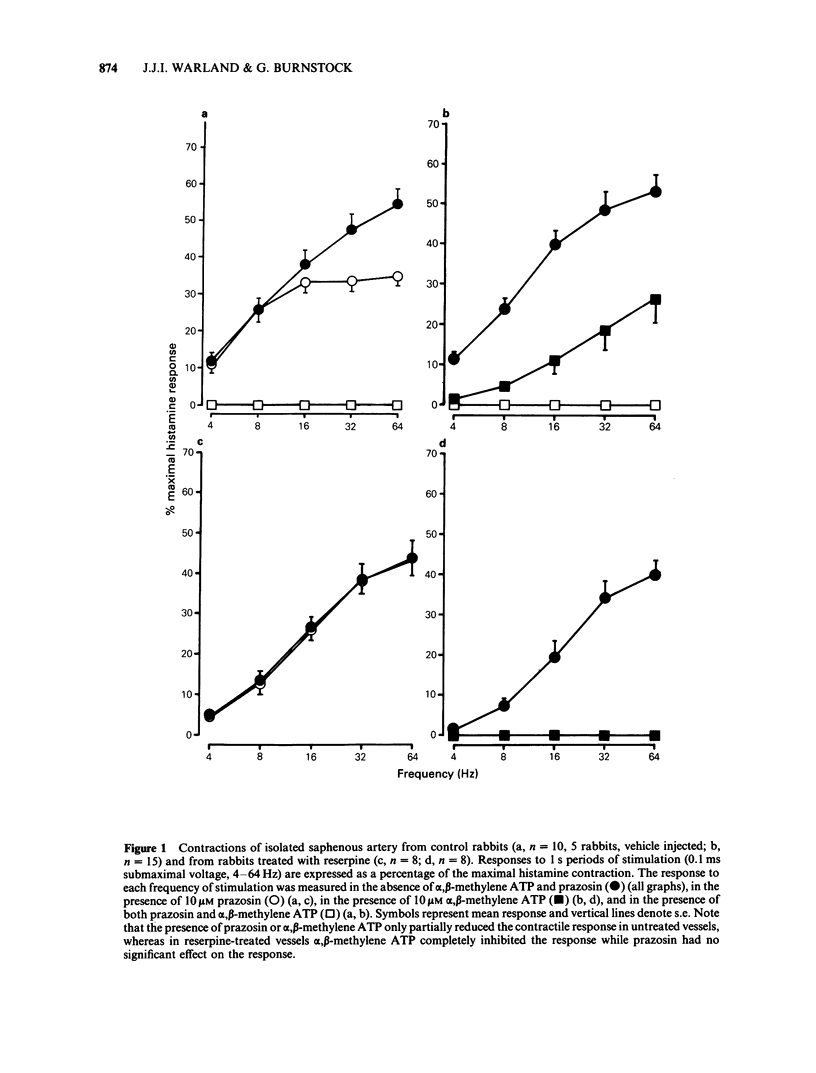
1 The effects of reserpine and of 6-hydroxydopamine on the contractions of the rabbit isolated saphenous artery produced by stimulation of the sympathetic nerves were studied. 2 In vessels exposed to reserpine, substantial contractions to nerve stimulation were recorded despite a 95.7% reduction in the noradrenaline content of the tissue. These responses of the vessel were not significantly affected by the alpha 1-antagonist, prazosin, whereas after desensitization of the P2-purinoceptor with alpha, beta-methylene ATP, no response to nerve stimulation remained. 3 In vessels exposed to 6-hydroxydopamine, no nerve-mediated responses were observed. 4 Noradrenaline-containing nerves were observed by fluorescence histochemistry in control tissues, but were not observed in tissues treated with reserpine or 6-hydroxydopamine. 5 The potencies of ATP and histamine were not significantly affected by reserpine or 6-hydroxydopamine treatment. However, there was a slight supersensitivity to noradrenaline in reserpine-treated and 6-hydroxydopamine-treated vessels compared with that of control vessels. Prazosin was selective for alpha-adrenoceptors, while alpha, beta-methylene ATP was selective for P2-purinoceptors. 6 These results substantiate the finding that ATP and noradrenaline are sympathetic cotransmitters in the rabbit isolated saphenous artery, and demonstrate that ATP can act as a transmitter independently of noradrenaline in this vessel.
Full text
PDF


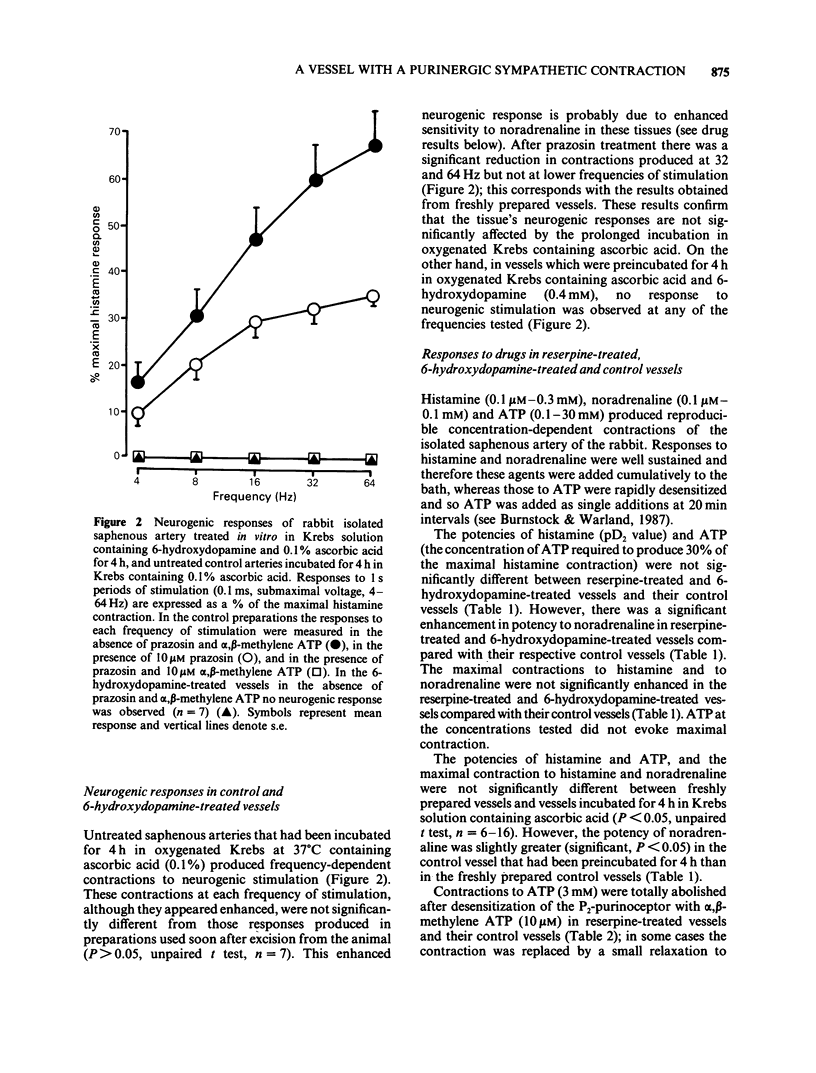




Selected References
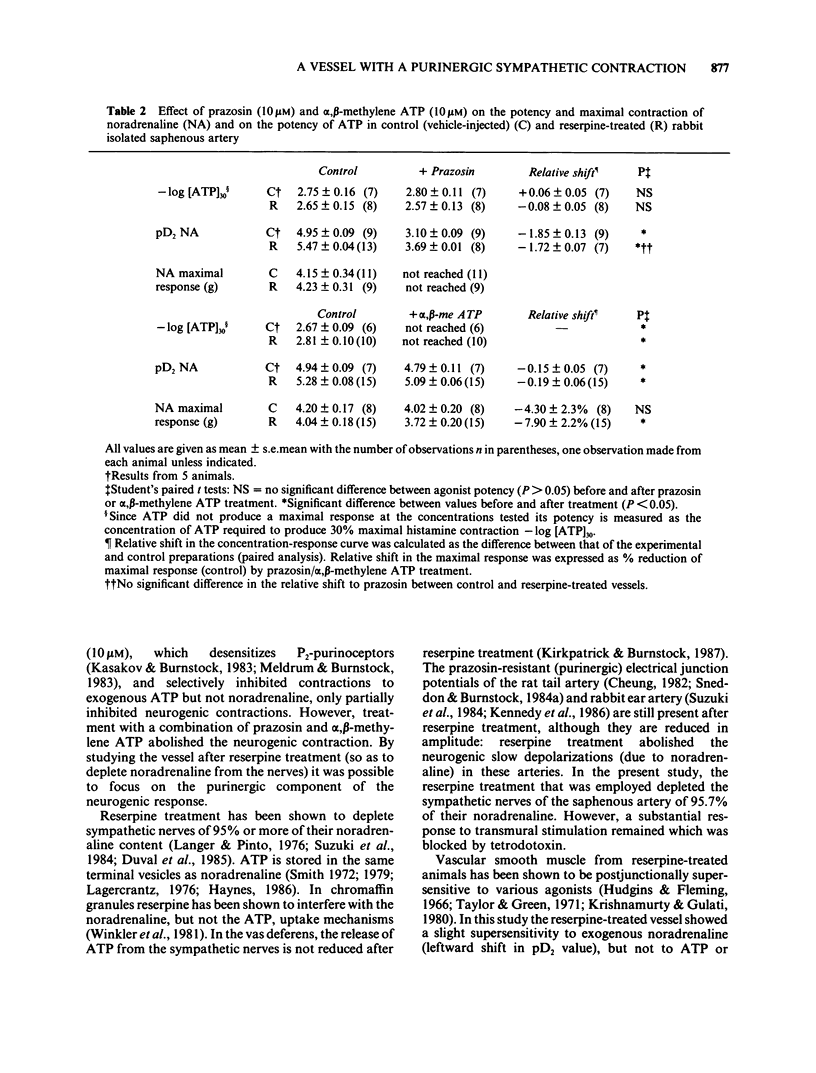
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Burnstock G., Cobb J. L., Malmfors T. An ultrastructural and histochemical study of the short-term effects of 6-hydroxydopamine on adrenergic nerves in the domestic fowl. Br J Pharmacol. 1970 Apr;38(4):802–809. doi: 10.1111/j.1476-5381.1970.tb09889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. A pharmacological study of the rabbit saphenous artery in vitro: a vessel with a large purinergic contractile response to sympathetic nerve stimulation. Br J Pharmacol. 1987 Jan;90(1):111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. The effect of alpha, beta-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in the immature rat basilar artery. Br J Pharmacol. 1986 May;88(1):6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W., Fujioka M. Inhibition of the excitatory junction potential in the guinea-pig saphenous artery by ANAPP3. Br J Pharmacol. 1986 Sep;89(1):3–5. doi: 10.1111/j.1476-5381.1986.tb11114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval N., Hicks P. E., Langer S. Z. Inhibitory effects of alpha, beta-methylene ATP on nerve-mediated contractions of the nictitating membrane in reserpinised cats. Eur J Pharmacol. 1985 Apr 16;110(3):373–377. doi: 10.1016/0014-2999(85)90567-9. [DOI] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z. Influence of reserpine-induced depletion of noradrenaline on the negative feed-back mechanism for transmitter release during nerve stimulation. Br J Pharmacol. 1973 Oct;49(2):214–225. doi: 10.1111/j.1476-5381.1973.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins P. M., Fleming W. W. A relatively nonspecific supersensitivity in aortic strips resulting from pretreatment with reserpine. J Pharmacol Exp Ther. 1966 Jul;153(1):70–80. [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Keller R., Oke A., Mefford I., Adams R. N. Liquid chromatographic analysis of catecholamines routine assay for regional brain mapping. Life Sci. 1976 Oct 1;19(7):995–1003. doi: 10.1016/0024-3205(76)90290-3. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Saville V. L., Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986 Apr 2;122(3):291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K., Burnstock G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur J Pharmacol. 1987 Jun 19;138(2):207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- Krishnamurty V. S., Gulati O. D. Influences of Mg++ and reserpine on calcium fluxes and sensitivity of the rat aorta. Arch Int Pharmacodyn Ther. 1980 Jul;246(1):61–70. [PubMed] [Google Scholar]
- Lagercrantz H. On the composition and function of large dense cored vesicles in sympathetic nerves. Neuroscience. 1976;1(2):81–92. doi: 10.1016/0306-4522(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Pinto J. E. Possible involvement of a transmitter different from norepinephrine in the residual responses to nerve stimulation of the cat nictitating membrane after pretreatment with reserpine. J Pharmacol Exp Ther. 1976 Mar;196(3):697–713. [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The glyoxylic acid fluorescence histochemical method: a detailed account of the methodology for the visualization of central catecholamine neurons. Histochemistry. 1974 Apr 22;39(2):97–127. doi: 10.1007/BF00492041. [DOI] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D. Subcellular localisation of noradrenaline in sympathetic neurons. Pharmacol Rev. 1972 Sep;24(3):435–457. [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985 Mar;14(3):929–946. doi: 10.1016/0306-4522(85)90155-1. [DOI] [PubMed] [Google Scholar]
- Taylor J., Green R. D. Analysis of reserpine-induced supersensitivity in aortic strips of rabbits. J Pharmacol Exp Ther. 1971 Apr;177(1):127–135. [PubMed] [Google Scholar]
- Thoenen H., Tranzer J. P. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-Hydroxydopamine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(3):271–288. doi: 10.1007/BF00536990. [DOI] [PubMed] [Google Scholar]
- Vidal M., Hicks P. E., Langer S. Z. Differential effects of alpha-beta-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):384–390. doi: 10.1007/BF00500092. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


