Abstract
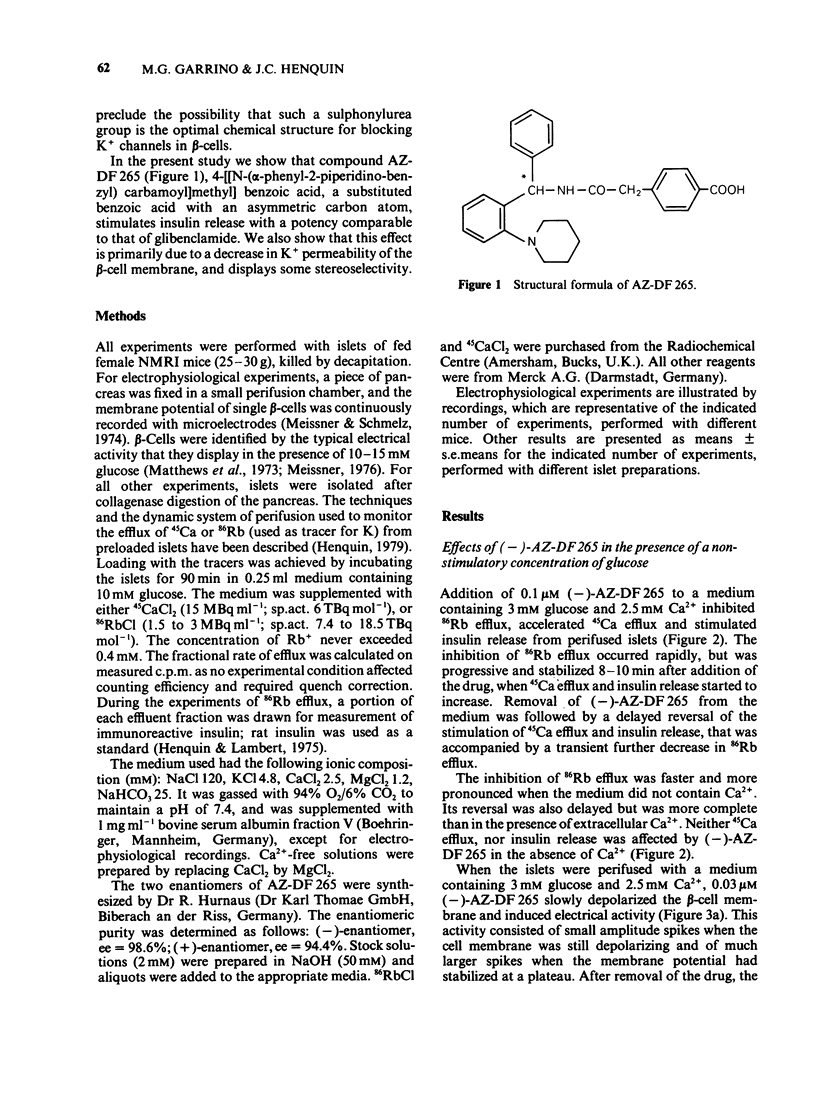
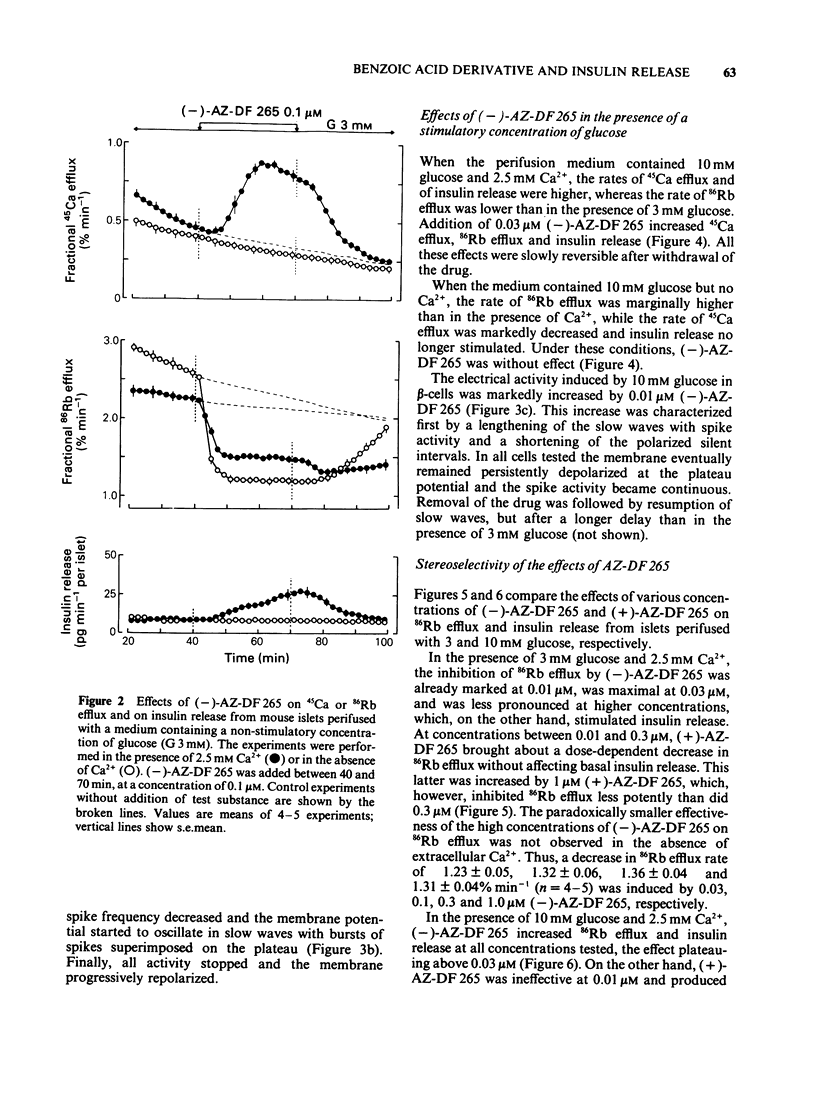
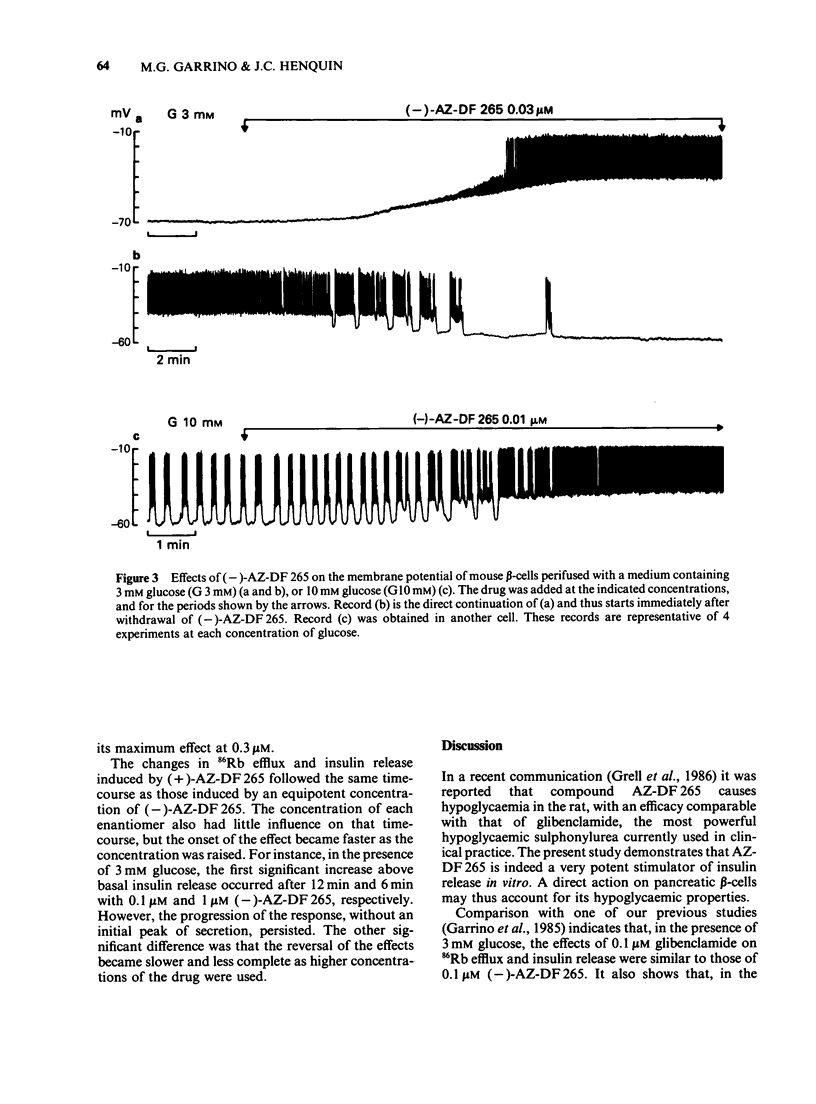
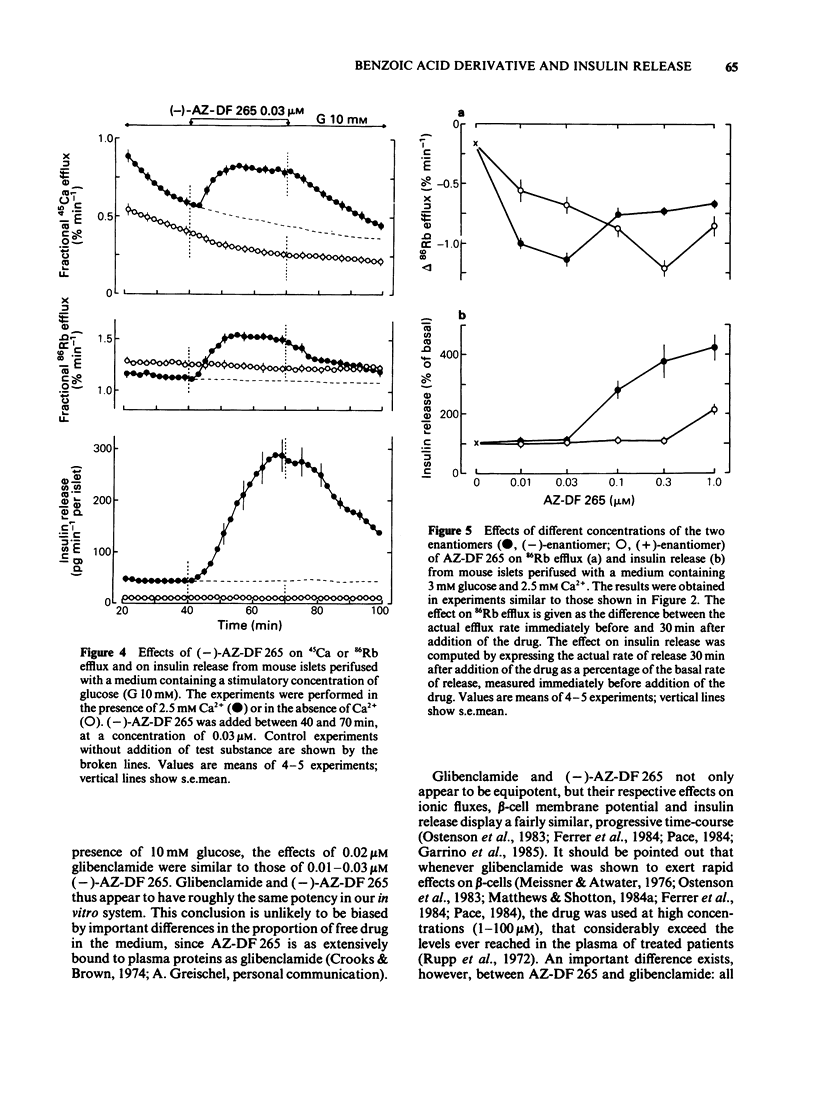
1. Mouse islets were used to define the characteristics and study the mechanisms of the stimulation of insulin release by compound AZ-DF 265, 4-[[N-(alpha-phenyl-2-piperidino-benzyl) carbamoyl]methyl] benzoic acid, a substituted benzoic acid with an asymmetric carbon atom. 2. At a non-stimulatory concentration of glucose (3 mM), (-)-AZ-DF 265 reversibly inhibited 86Rb efflux from islet cells, depolarized the beta-cell membrane, induced electrical activity, stimulated 45Ca efflux, and triggered insulin release. Maximum inhibition of 86Rb efflux occurred at 0.03 microM (-)-AZ-DF 265, whereas the threshold concentration for stimulation of release was 0.1 microM. Omission of extracellular Ca2+ abolished all effects of the drug but the inhibition of 86Rb efflux. 3. At a stimulatory concentration of glucose (10 mM), (-)-AZ-DF 265 reversibly increased 86Rb efflux, potentiated electrical activity, augmented 45Ca efflux, and increased insulin release. Maximum stimulation of 86Rb efflux and insulin release was obtained with 0.03 microM (-)-AZ-DF 265. Omission of extracellular Ca2+ abolished all effects of the drug. 4. The potency of (-)-AZ-DF 265 was similar to that of glibenclamide, whereas the (+)-enantiomer was about 10 times less potent on 86Rb efflux and insulin release. 5. It is concluded that, like sulphonylureas, compound AZ-DF 265 decreases K+ permeability of the beta-cell membrane and thereby causes depolarization. This activates voltage-dependent Ca channels, permits Ca2+ influx and eventually stimulates insulin release. Its stereoselectivity may help to elucidate the mechanisms of K channel blockade and, hence, lead to the design of more potent and specific insulinotropic drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H., Berggren P. O., Rorsman P. Direct measurements of increased free cytoplasmic Ca2+ in mouse pancreatic beta-cells following stimulation by hypoglycemic sulfonylureas. FEBS Lett. 1985 Oct 7;190(1):21–24. doi: 10.1016/0014-5793(85)80418-x. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Crooks M. J., Brown K. F. The binding of sulphonylureas to serum albumin. J Pharm Pharmacol. 1974 May;26(5):304–311. doi: 10.1111/j.2042-7158.1974.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Deleers M., Malaisse W. J. Binding of hypoglycaemic sulphonylureas to an artificial phospholipid bilayer. Diabetologia. 1984 Jan;26(1):55–59. doi: 10.1007/BF00252264. [DOI] [PubMed] [Google Scholar]
- Ferrer R., Atwater I., Omer E. M., Gonçalves A. A., Croghan P. C., Rojas E. Electrophysiological evidence for the inhibition of potassium permeability in pancreatic beta-cells by glibenclamide. Q J Exp Physiol. 1984 Oct;69(4):831–839. doi: 10.1113/expphysiol.1984.sp002872. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium. J Membr Biol. 1985;83(1-2):169–175. doi: 10.1007/BF01868748. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Meissner H. P., Henquin J. C. The non-sulfonylurea moiety of gliquidone mimics the effects of the parent molecule on pancreatic B-cells. Eur J Pharmacol. 1986 May 27;124(3):309–316. doi: 10.1016/0014-2999(86)90232-3. [DOI] [PubMed] [Google Scholar]
- Gylfe E., Hellman B., Sehlin J., Täljedal B. Interaction of sulfonylurea with the pancreatic B-cell. Experientia. 1984 Oct 15;40(10):1126–1134. doi: 10.1007/BF01971460. [DOI] [PubMed] [Google Scholar]
- Hellman B. Tolbutamide stimulation of 45Ca fluxes in microdissected pancreatic islets rich in beta-cells. Mol Pharmacol. 1981 Jul;20(1):83–88. [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia. 1980;18(2):151–160. doi: 10.1007/BF00290493. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Shotton P. A. Efflux of 86Rb from rat and mouse pancreatic islets: the role of membrane depolarization. Br J Pharmacol. 1984 Nov;83(3):831–839. doi: 10.1111/j.1476-5381.1984.tb16239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Shotton P. A. The control of 86Rb efflux from rat isolated pancreatic islets by the sulphonylureas tolbutamide and glibenclamide. Br J Pharmacol. 1984 Jul;82(3):689–700. doi: 10.1111/j.1476-5381.1984.tb10808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon R. E., Marshall F. J., Culp H. W. The nature of the metabolites of acetohexamide in the rat and in the human. J Pharmacol Exp Ther. 1965 Aug;149(2):272–279. [PubMed] [Google Scholar]
- Meissner H. P., Atwater I. J. The kinetics of electrical activity of beta cells in response to a "square wave" stimulation with glucose or glibenclamide. Horm Metab Res. 1976 Jan;8(1):11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Meissner H. P. Electrical characteristics of the beta-cells in pancreatic islets. J Physiol (Paris) 1976 Nov;72(6):757–767. [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Ostenson C. G., Grill V., Nylén A., Efendić S. Modulation by IBMX, fasting and experimental diabetes of glibenclamide-induced islet hormone release from the perfused rat pancreas. Diabete Metab. 1983 Mar;9(1):58–65. [PubMed] [Google Scholar]
- Pace C. S. Influence of a tumor-promoting phorbol ester on the electrical response of B-cells to glucose and glyburide. Mol Pharmacol. 1984 Sep;26(2):267–271. [PubMed] [Google Scholar]
- Rufer C., Biere H., Ahrens H., Loge O., Schröder E. Blood glucose lowering sulfonamides with asymmetric carbon atoms. J Med Chem. 1974 Jul;17(7):708–715. doi: 10.1021/jm00253a010. [DOI] [PubMed] [Google Scholar]
- Rufer C., Losert W. Blood glucose lowering sulfonamides with asymmetric carbon atoms. 3. Related N-substituted carbamoylbenzoic acids. J Med Chem. 1979 Jun;22(6):750–752. doi: 10.1021/jm00192a028. [DOI] [PubMed] [Google Scholar]
- Rupp W., Christ O., Fülberth W. Untersuchungen zur Bioavailability von Glibenclamid. Arzneimittelforschung. 1972 Feb;22(2):471–473. [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Hales C. N., Ashford M. L. Inhibition of a calcium-activated, non-selective cation channel, in a rat insulinoma cell line, by adenine derivatives. FEBS Lett. 1986 Nov 24;208(2):397–400. doi: 10.1016/0014-5793(86)81056-0. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]


