Abstract
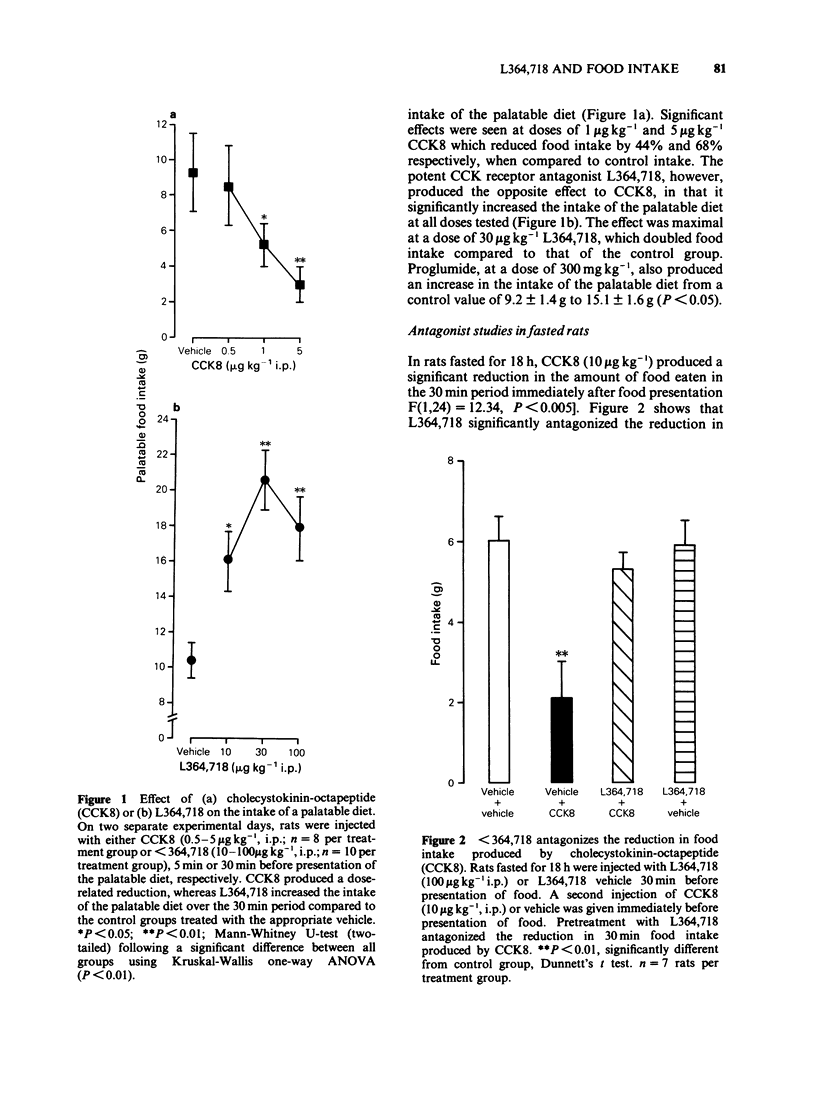
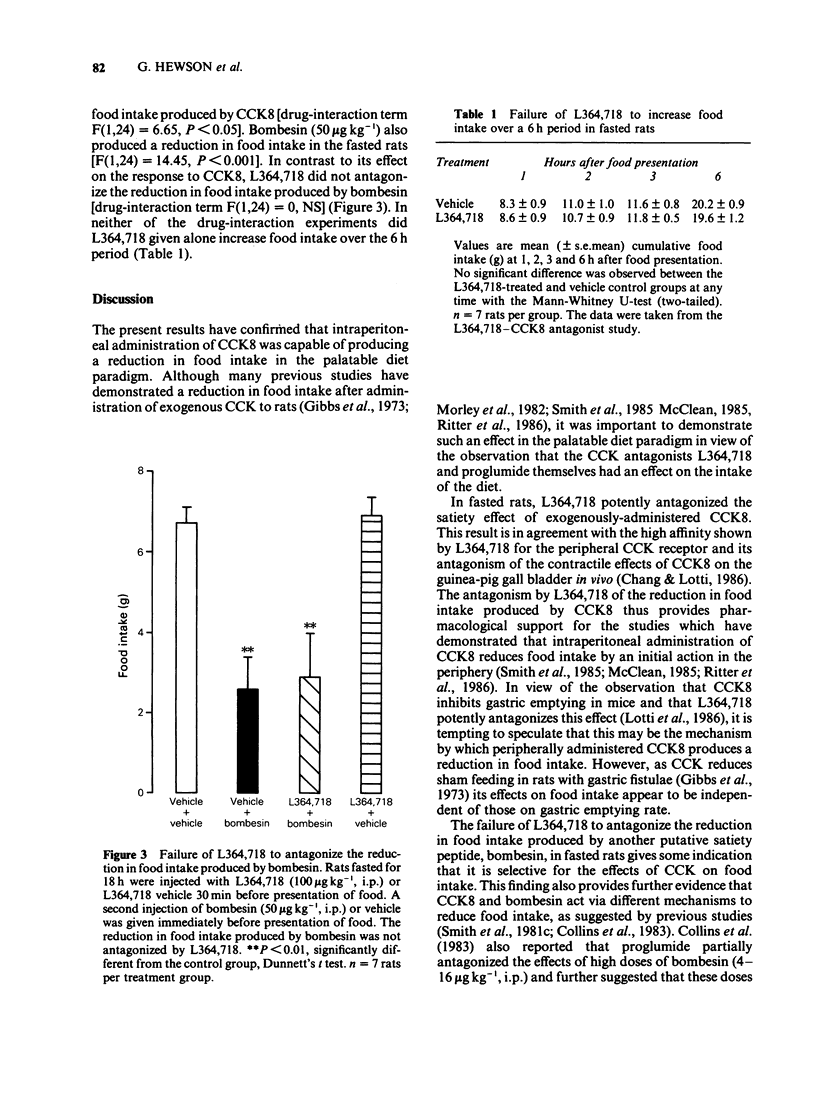
1. To determine the role of endogenous cholecystokinin (CCK) in the regulation of food intake, the effects of the potent CCK receptor antagonist L364,718 were investigated on the intake of a palatable diet in non-deprived rats. The effect of a single dose of proglumide was also investigated for comparative purposes. In addition, the ability of L364,718 to antagonize the reduction in food intake produced by exogenous cholecystokinin-octapeptide (CCK8) or bombesin in food-deprived rats was determined. 2. L364,718 (10-100 micrograms kg-1, i.p.) increased the intake of palatable diet during the 30 min test period. Proglumide (300 mg kg-1, i.p.) also increased the intake of palatable diet. Conversely, CCK8 (0.5-5 micrograms kg-1, i.p.) produced a reduction in the intake of the diet. 3. In fasted rats, L364,718 (100 micrograms kg-1, i.p.) antagonized the reduction in food intake produced by CCK8 (10 micrograms kg-1, i.p.) but not that produced by bombesin (50 micrograms kg-1, i.p.). L364,718 did not increase food intake in these animals when measured over a 6 h period. 4. It is concluded that L364,718 is a potent, selective antagonist of the effects of CCK8 on food intake. The observation that L364,718 and proglumide increase the intake of a palatable diet provides some evidence that endogenous CCK is involved in the control of food intake in this model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burhol P. G., Jenssen T. G., Jorde R., Lygren I., Johnson J. A. Plasma cholecystokinin (CCK) before and after a jejunoileal bypass operation in obese patients with reference to appetite regulation. Int J Obes. 1984;8(3):233–236. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Monaghan R. L., Birnbaum J., Stapley E. O., Goetz M. A., Albers-Schönberg G., Patchett A. A., Liesch J. M., Hensens O. D. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- Collins S., Walker D., Forsyth P., Belbeck L. The effects of proglumide on cholecystokinin-, bombesin-, and glucagon-induced satiety in the rat. Life Sci. 1983 May 9;32(19):2223–2229. doi: 10.1016/0024-3205(83)90420-4. [DOI] [PubMed] [Google Scholar]
- Cooper S. J., Moores W. R., Jackson A., Barber D. J. Effects of tifluadom on food consumption compared with chlordiazepoxide and kappa agonists in the rat. Neuropharmacology. 1985 Sep;24(9):877–883. doi: 10.1016/0028-3908(85)90039-5. [DOI] [PubMed] [Google Scholar]
- Cooper S. J., Yerbury R. E. Benzodiazepine-induced hyperphagia: stereospecificity and antagonism by pyrazoloquinolines, CGS 9895 and CGS 9896. Psychopharmacology (Berl) 1986;89(4):462–466. doi: 10.1007/BF02412122. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Stivers J. A., Hommer D. W., Skirboll L. R., Paul S. M. Antagonists of central and peripheral behavioral actions of cholecystokinin octapeptide. J Pharmacol Exp Ther. 1986 Feb;236(2):320–330. [PubMed] [Google Scholar]
- Evans B. E., Bock M. G., Rittle K. E., DiPardo R. M., Whitter W. L., Veber D. F., Anderson P. S., Freidinger R. M. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4918–4922. doi: 10.1073/pnas.83.13.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J., Young R. C., Smith G. P. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973 Sep;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Hahne W. F., Jensen R. T., Lemp G. F., Gardner J. D. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Lotti V. J., Cerino D. J., Kling P. J., Change R. S. A new simple mouse model for the in vivo evaluation of cholecystokinin (CCK) antagonists: comparative potencies and durations of action of nonpeptide antagonists. Life Sci. 1986 Nov 3;39(18):1631–1638. doi: 10.1016/0024-3205(86)90159-1. [DOI] [PubMed] [Google Scholar]
- Morley J. E., Levine A. S., Kneip J., Grace M. The effect of vagotomy on the satiety effects of neuropeptides and naloxone. Life Sci. 1982 May 31;30(22):1943–1947. doi: 10.1016/0024-3205(82)90476-3. [DOI] [PubMed] [Google Scholar]
- Ritter R. C., Kalivas P., Bernier S. Cholecystokinin-induced suppression of locomotion is attenuated in capsaicin pretreated rats. Peptides. 1986 Jul-Aug;7(4):587–590. doi: 10.1016/0196-9781(86)90031-8. [DOI] [PubMed] [Google Scholar]
- Schneider L. H., Gibbs J., Smith G. P. Proglumide fails to increase food intake after an ingested preload. Peptides. 1986 Jan-Feb;7(1):135–140. doi: 10.1016/0196-9781(86)90073-2. [DOI] [PubMed] [Google Scholar]
- Shillabeer G., Davison J. S. The cholecystokinin antagonist, proglumide, increases food intake in the rat. Regul Pept. 1984 Apr;8(3):171–176. doi: 10.1016/0167-0115(84)90058-2. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Jerome C., Cushin B. J., Eterno R., Simansky K. J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981 Aug 28;213(4511):1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Jerome C., Gibbs J. Abdominal vagotomy does not block the satiety effect of bombesin in the rat. Peptides. 1981 Winter;2(4):409–411. doi: 10.1016/s0196-9781(81)80096-4. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Jerome C., Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985 Nov;249(5 Pt 2):R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]


