Abstract
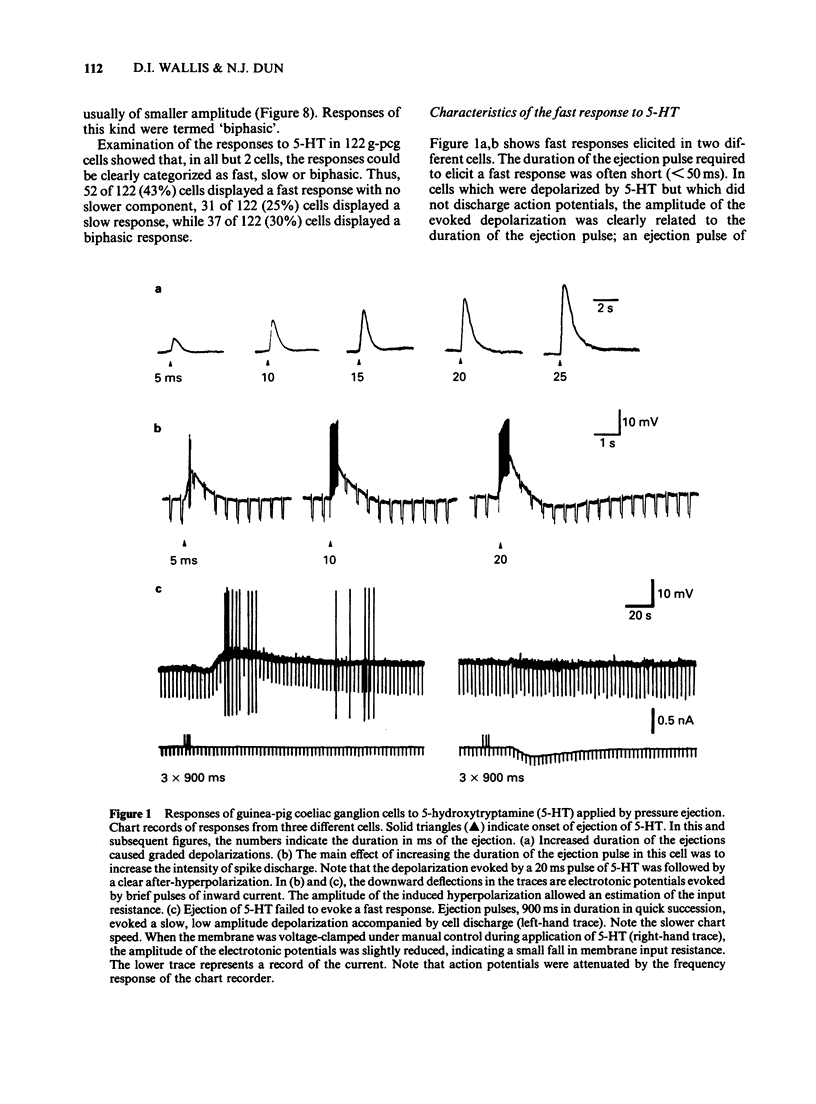
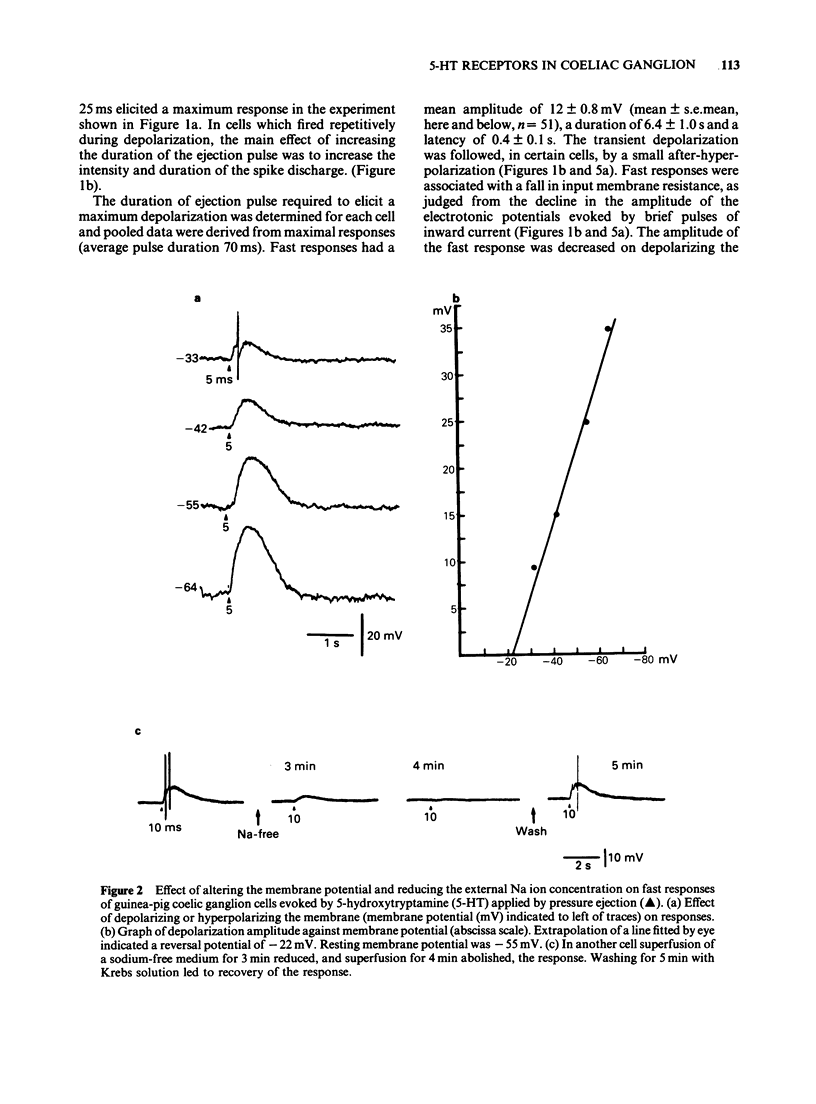
1. 5-Hydroxytryptamine (5-HT) was applied by pressure ejection to coeliac ganglion cells of the guinea-pig maintained in vitro and responses measured intracellularly. 2. Cells responded in one of three ways to 5-HT: by (a) a fast, transient depolarization (43%), (b) a fast transient followed by a slow depolarization (biphasic response, 30%) or (c) a slow sustained depolarization (25%). 3. Fast depolarizations (response (a) above] were graded according to the duration of the ejection pulse. Maximal responses had a mean amplitude of 12 +/- 0.8 mV, a duration of 6.4 +/- 1.0 s, a latency of 0.4 +/- 0.1 s, were associated with a fall in membrane input resistance, increased in amplitude by hyperpolarization and probably mediated by an increased conductance to Na and K. The estimated reversal potential was -22.8 +/- 2.4 mV (n = 14). The maximal fast response seen in biphasically-responding cells (b) appeared similar to fast response (a). 4. Fast depolarizations (a) showed marked tachyphylaxis and were abolished by superfusion of the ganglion with 5-HT (100 microM). They were reduced in amplitude by tubocurarine (10-100 microM, pIC50 4.4), MDL 72222 (1-5 microM, pIC50 5.8), quipazine (1 microM reduced responses by 65 +/- 15%, n = 3), ICS 205-930 (1 microM reduced responses by 64 +/- 14%, n = 7) and metoclopramide (10 microM reduced responses by about 45%), but were unafected by methysergide (up to 1 microM) or hexamethonium (up to 1 mM). 5. Slow depolarizations (c) varied in amplitude with the duration of the ejection pulse. Maximal responses had a mean amplitude of 6.4 +/- 0.7 mV, a duration of 62 +/- 6 s, a latency of 3.5 +/- 0.8 s and were reduced in amplitude by methysergide (0.1-1 microM, pIC50 6.5) but not by MDL 72222 (1 microM). The maximal slow component in biphasically-responding cells (b) was similar in amplitude and duration to slow response (c), was partially blocked by methysergide (1-5 microM) in 4 of 6 cells and was enhanced by tubocurarine (50 microM) which reduced the fast component. 6. Slow depolarizations (b,c) were associated with either a small reduction or no change in membrane input resistance depending on the cell studied. Hyperpolarization had variable effects on slow depolarization amplitude.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

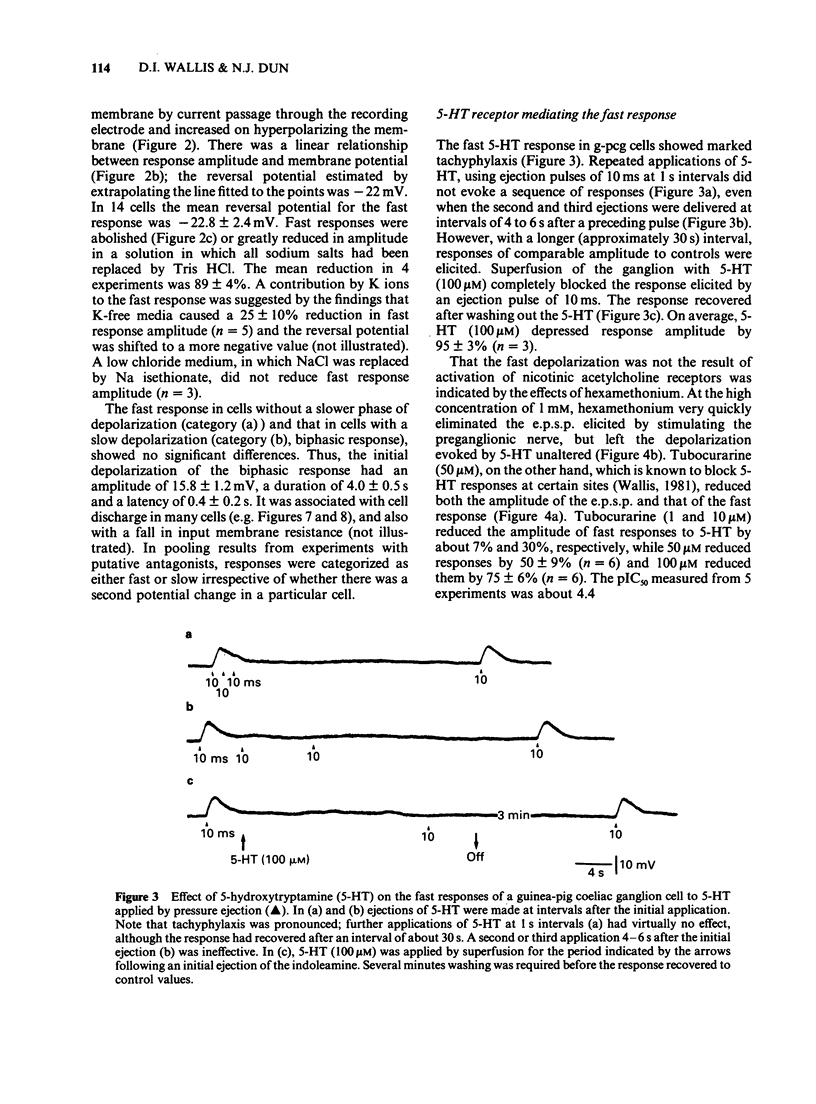
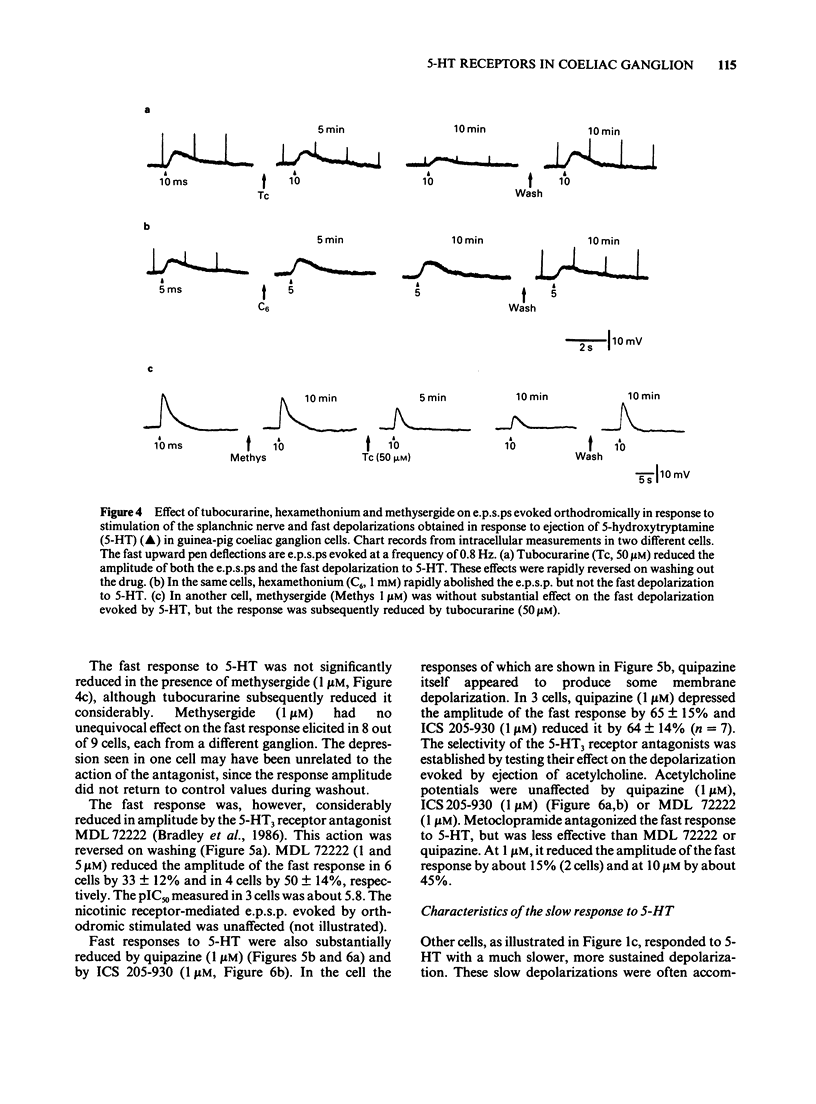
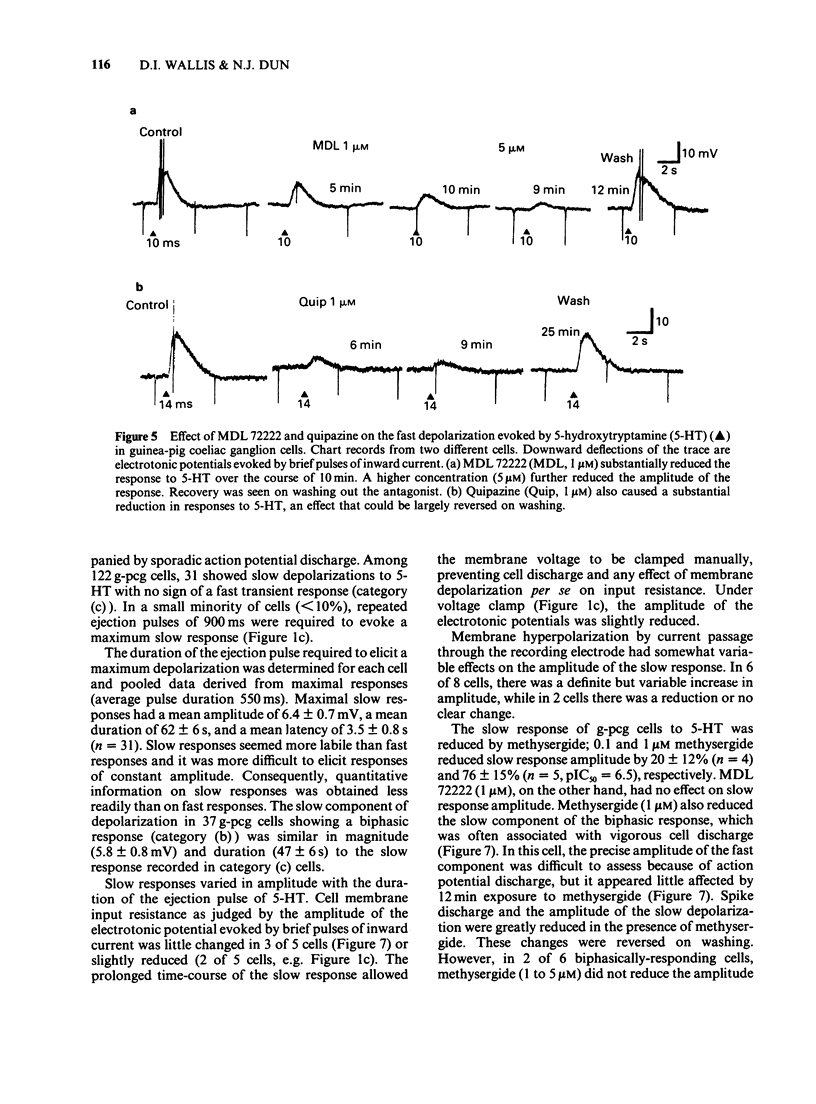
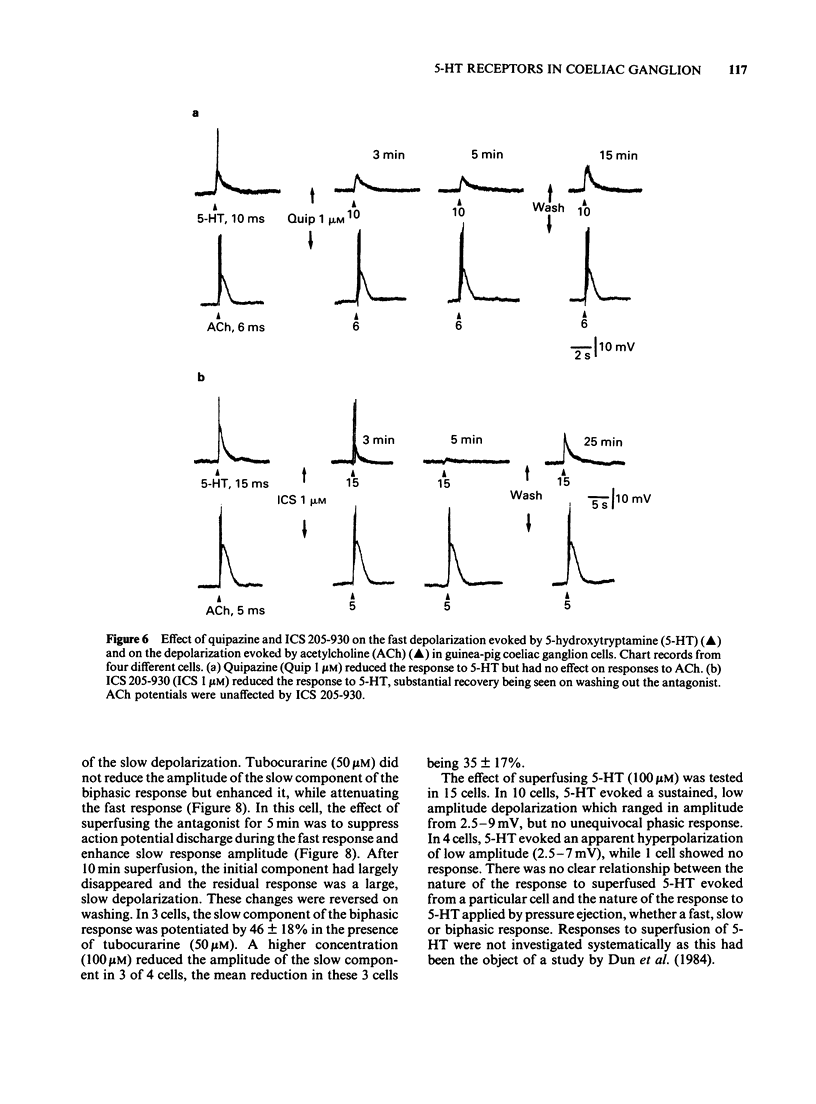
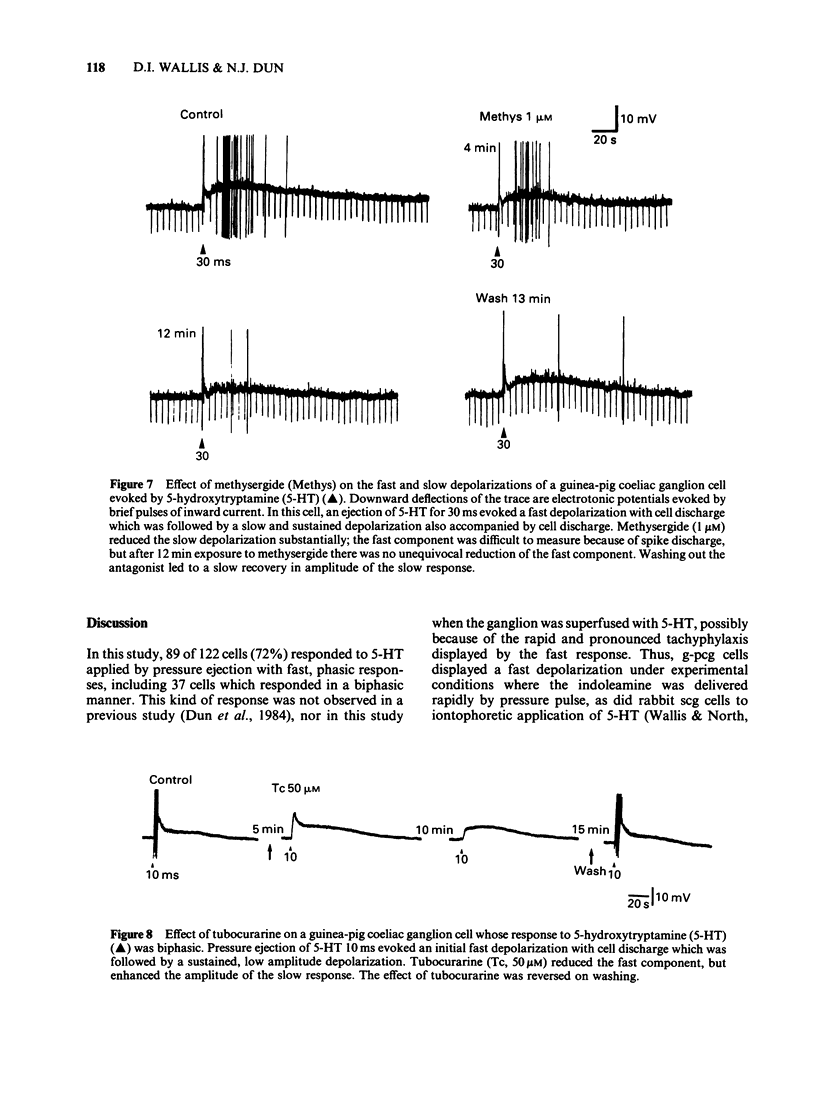


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azami J., Fozard J. R., Round A. A., Wallis D. I. The depolarizing action of 5-hydroxytryptamine on rabbit vagal primary afferent and sympathetic neurones and its selective blockade by MDL 72222. Naunyn Schmiedebergs Arch Pharmacol. 1985 Feb;328(4):423–429. doi: 10.1007/BF00692911. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Kiraly M., Ma R. C. Evidence for a serotonin-mediated slow excitatory potential in the guinea-pig coeliac ganglia. J Physiol. 1984 Jun;351:61–76. doi: 10.1113/jphysiol.1984.sp015232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Ma R. C. Slow non-cholinergic excitatory potentials in neurones of the guinea-pig coeliac ganglia. J Physiol. 1984 Jun;351:47–60. doi: 10.1113/jphysiol.1984.sp015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984 Dec;23(12B):1473–1486. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Multiple actions of 5-hydroxytryptamine on myenteric neurones of the guinea-pig ileum. J Physiol. 1980 Jul;304:459–470. doi: 10.1113/jphysiol.1980.sp013336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R. C., Horwitz J., Kiraly M., Perlman R. L., Dun N. J. Immunohistochemical and biochemical detection of serotonin in the guinea pig celiac-superior mesenteric plexus. Neurosci Lett. 1985 May 14;56(2):107–112. doi: 10.1016/0304-3940(85)90115-6. [DOI] [PubMed] [Google Scholar]
- Mawe G. M., Branchek T. A., Gershon M. D. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9799–9803. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. L., Wallis D. I., Ash G. 5-HT antagonists and blockade of neuronal (5-HT) receptors on ganglion cells. Gen Pharmacol. 1984;15(4):339–344. doi: 10.1016/0306-3623(84)90011-9. [DOI] [PubMed] [Google Scholar]
- Richardson B. P., Engel G., Donatsch P., Stadler P. A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985 Jul 11;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Round A., Wallis D. I. Further studies on the blockade of 5-HT depolarizations of rabbit vagal afferent and sympathetic ganglion cells by MDL 72222 and other antagonists. Neuropharmacology. 1987 Jan;26(1):39–48. doi: 10.1016/0028-3908(87)90042-6. [DOI] [PubMed] [Google Scholar]
- Round A., Wallis D. I. The depolarizing action of 5-hydroxytryptamine on rabbit vagal afferent and sympathetic neurones in vitro and its selective blockade by ICS 205-930. Br J Pharmacol. 1986 Jun;88(2):485–494. doi: 10.1111/j.1476-5381.1986.tb10227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Woodward B. Membrane potential changes induced by 5-hydroxytryptamine in the rabbit superior cervical ganglion. Br J Pharmacol. 1975 Oct;55(2):199–212. doi: 10.1111/j.1476-5381.1975.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. Neuronal 5-hydroxytryptamine receptors outside the central nervous system. Life Sci. 1981 Dec 7;29(23):2345–2355. doi: 10.1016/0024-3205(81)90470-7. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]


