Abstract
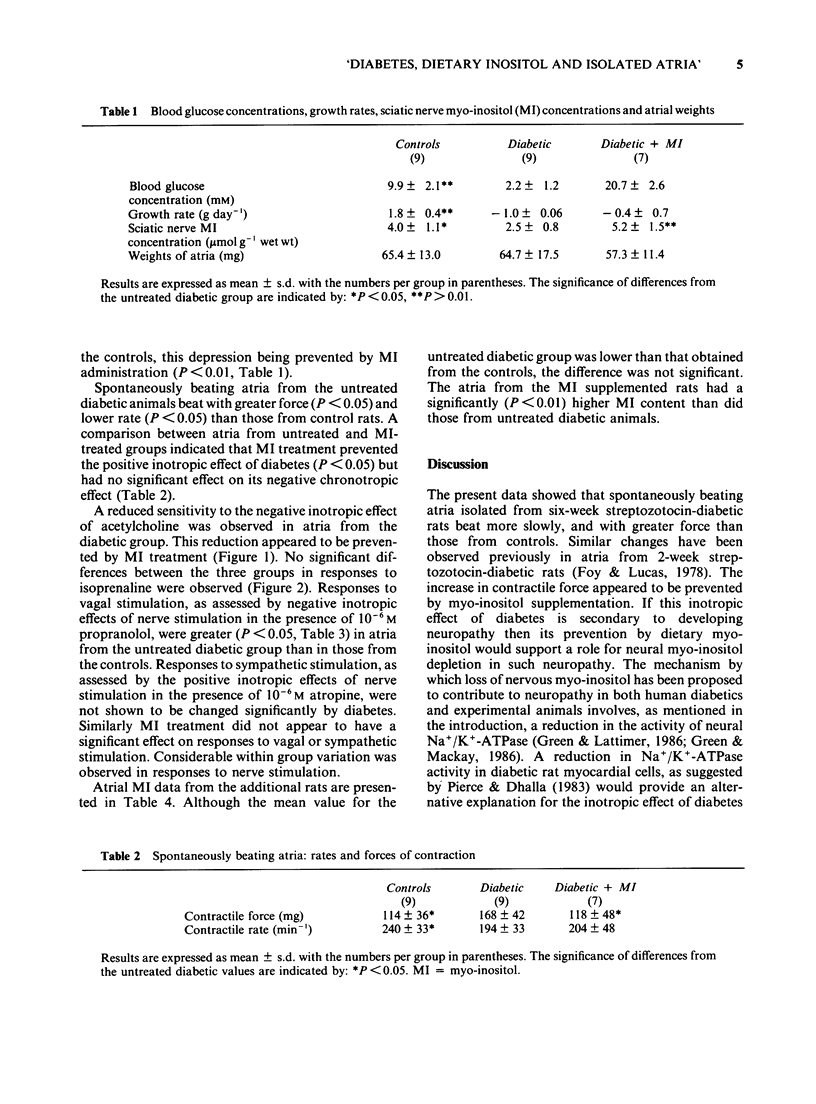
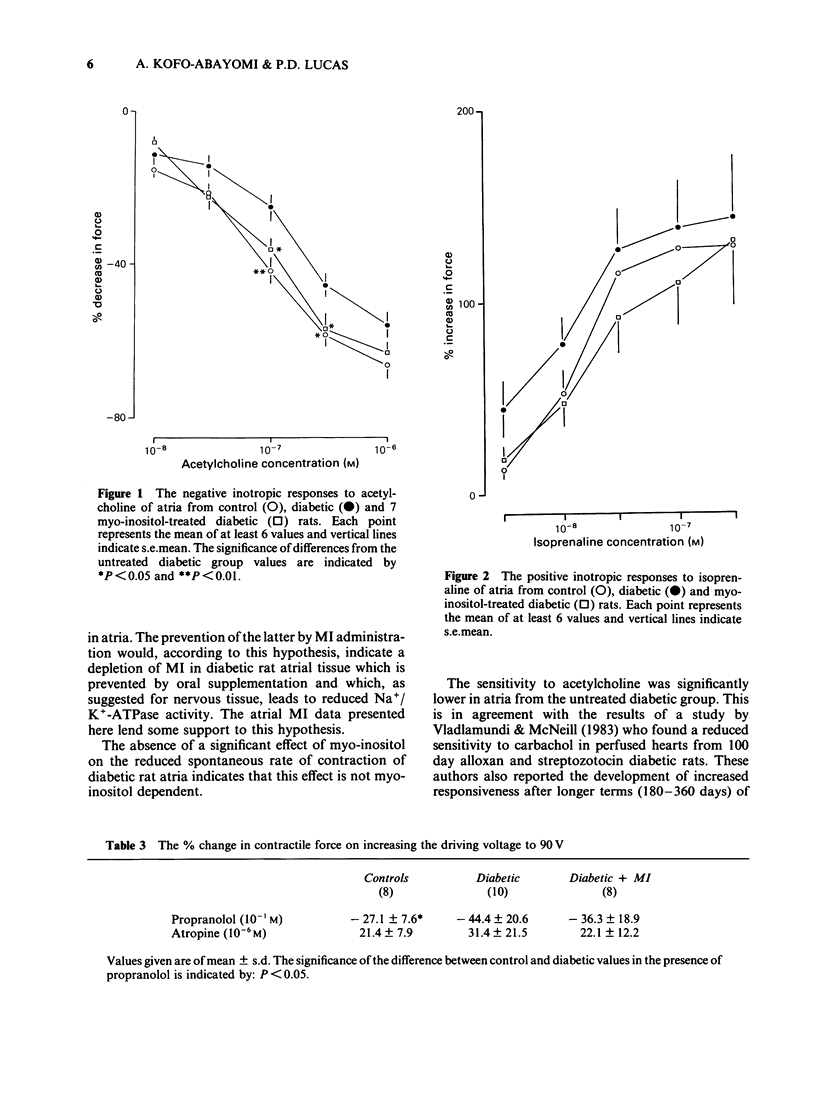
1. Atria, isolated from control rats, six-week streptozotocin-diabetic rats and from similarly diabetic rats treated with myo-inositol (MI) were compared. The MI treatment was shown to reverse the depressed sciatic nerve MI which was observed in the untreated diabetic group. 2. Spontaneously beating atria from the untreated diabetic animals beat more slowly, and with greater force than tissues from the control group. When electrically driven at 4 Hz they were found to be less sensitive to the negative inotropic effect of acetylcholine. No differences between the two groups were observed in responses to isoprenaline. 3. Intramural nerve stimulation in the presence of 10(-6)M propranolol (vagal stimulation) had a greater negative inotropic effect in the untreated diabetic rat atria than in the controls. Positive inotropic responses to nerve stimulation in the presence of 10(-6) M atropine (sympathetic stimulation) were not significantly different between the two groups. 4. Atria from the MI-treated diabetic animals were found to have a lower spontaneous contractile force and greater sensitivity to acetylcholine than tissues from the untreated diabetic animals. The values obtained in both cases were similar to those from the controls. No significant effect of MI treatment on spontaneous contractile rate or on responses to nerve stimulation was demonstrated. 5. Atrial (mainly myocardial) MI was measured in additional control, six-week diabetic and six-week MI-treated diabetic animals. A significantly higher concentration was observed in the MI supplemented group compared to the untreated diabetic group. The mean MI content in the latter group was lower than that obtained from control tissues but not significantly so.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke B. F., Ewing D. J., Campbell I. W. Diabetic autonomic neuropathy. Diabetologia. 1979 Oct;17(4):195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- Foy J. M., Lucas P. D. Comparison between spontaneously beating atria from control and streptozocin-diabetic rats. J Pharm Pharmacol. 1978 Sep;30(9):558–562. doi: 10.1111/j.2042-7158.1978.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Foy J. M., Lucas P. D. Effect of experimental diabetes, food deprivation and genetic obesity on the sensitivity of pithed rats to autonomic agents. Br J Pharmacol. 1976 Jun;57(2):229–234. doi: 10.1111/j.1476-5381.1976.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galper J. B., Smith T. W. Agonist and guanine nucleotide modulation of muscarinic cholinergic receptors in cultured heart cells. J Biol Chem. 1980 Oct 25;255(20):9571–9579. [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N., Tomlinson D. R. Myo-inositol and sorbitol metabolism in relation to peripheral nerve function in experimental diabetes in the rat: the effect of aldose reductase inhibition. Diabetologia. 1983 Oct;25(4):365–371. doi: 10.1007/BF00253203. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Mackway A. M. Decreased myo-inositol content and Na+-K+-ATPase activity in superior cervical ganglion of STZ-diabetic rat and prevention by aldose reductase inhibition. Diabetes. 1986 Oct;35(10):1106–1108. doi: 10.2337/diab.35.10.1106. [DOI] [PubMed] [Google Scholar]
- Latifpour J., McNeill J. H. Cardiac autonomic receptors: effect of long-term experimental diabetes. J Pharmacol Exp Ther. 1984 Jul;230(1):242–249. [PubMed] [Google Scholar]
- Lloyd-Mostyn R. H., Watkins P. J. Defective innervation of heart in diabetic autonomic neuropathy. Br Med J. 1975 Jul 5;3(5974):15–17. doi: 10.1136/bmj.3.5974.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Schmelzer J. D., Ward K. K., Yao J. K. Experimental chronic hypoxic neuropathy: relevance to diabetic neuropathy. Am J Physiol. 1986 Jan;250(1 Pt 1):E94–E99. doi: 10.1152/ajpendo.1986.250.1.E94. [DOI] [PubMed] [Google Scholar]
- Magyar K., Nguyen T. T., Török T. L., Tóth P. T. The action of excess potassium and calcium on ouabain-evoked [3H]-noradrenaline release from the rabbit pulmonary artery. Br J Pharmacol. 1986 Jan;87(1):63–71. doi: 10.1111/j.1476-5381.1986.tb10157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R., McLean W. G. Slow orthograde axonal transport of radiolabelled protein in sciatic motoneurones of rats with short-term experimental diabetes: effects of treatment with an aldose reductase inhibitor or myo-inositol. J Neurochem. 1984 Nov;43(5):1265–1270. doi: 10.1111/j.1471-4159.1984.tb05382.x. [DOI] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Dhalla N. S. Sarcolemmal Na+-K+-ATPase activity in diabetic rat heart. Am J Physiol. 1983 Sep;245(3):C241–C247. doi: 10.1152/ajpcell.1983.245.3.C241. [DOI] [PubMed] [Google Scholar]
- Stuesse S. L., Wallick D. W., Mace S. Vagal control of heart period in alloxan diabetic rats. Life Sci. 1982 Jul 26;31(4):393–398. doi: 10.1016/0024-3205(82)90420-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson D. R., Yusof A. P. Autonomic neuropathy in the alloxan-diabetic rat. J Auton Pharmacol. 1983 Dec;3(4):257–263. doi: 10.1111/j.1474-8673.1983.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. V., McNeill J. H. Effect of alloxan- and streptozotocin-induced diabetes on isolated rat heart responsiveness to carbachol. J Pharmacol Exp Ther. 1983 May;225(2):410–415. [PubMed] [Google Scholar]
- Vizi E. S., Torok T., Seregi A., Serfozo P., Adam-Vizi V. Na-K activated ATPase and the release of acetylcholine and noradrenaline. J Physiol (Paris) 1982;78(4):399–406. [PubMed] [Google Scholar]
- Wheeler T., Watkins P. J. Cardiac denervation in diabetes. Br Med J. 1973 Dec 8;4(5892):584–586. doi: 10.1136/bmj.4.5892.584. [DOI] [PMC free article] [PubMed] [Google Scholar]



