Abstract
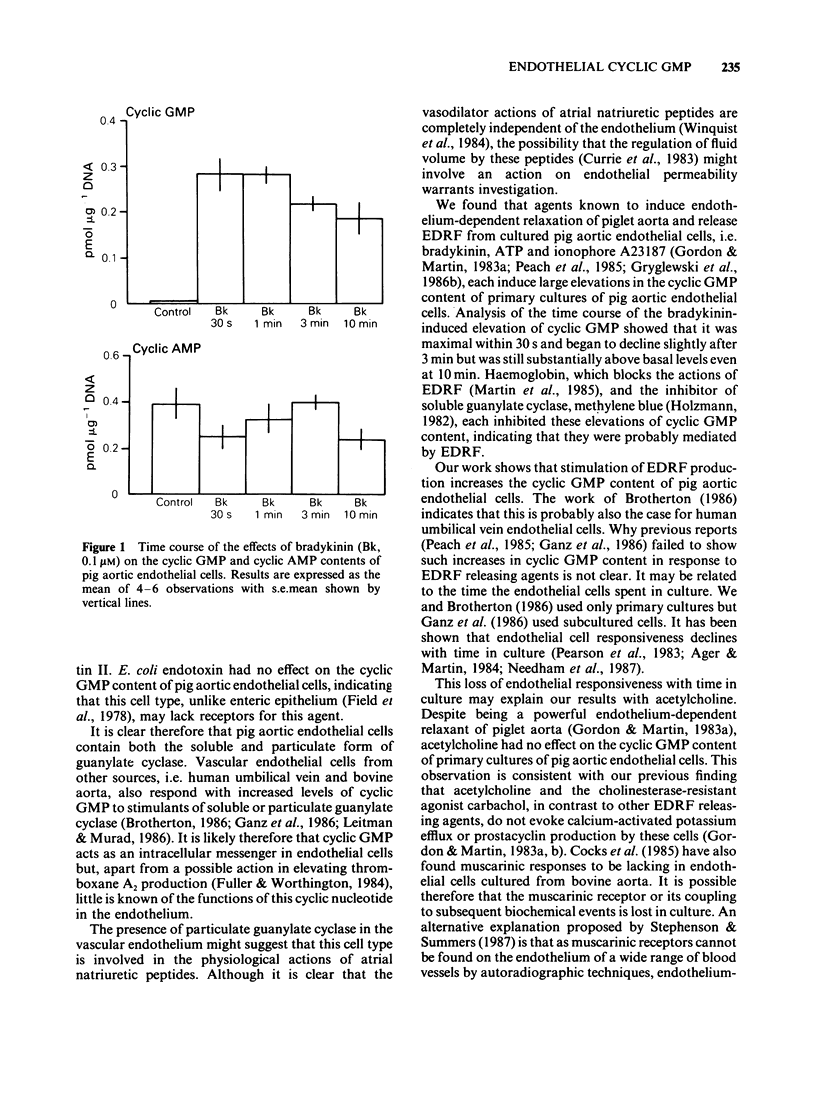
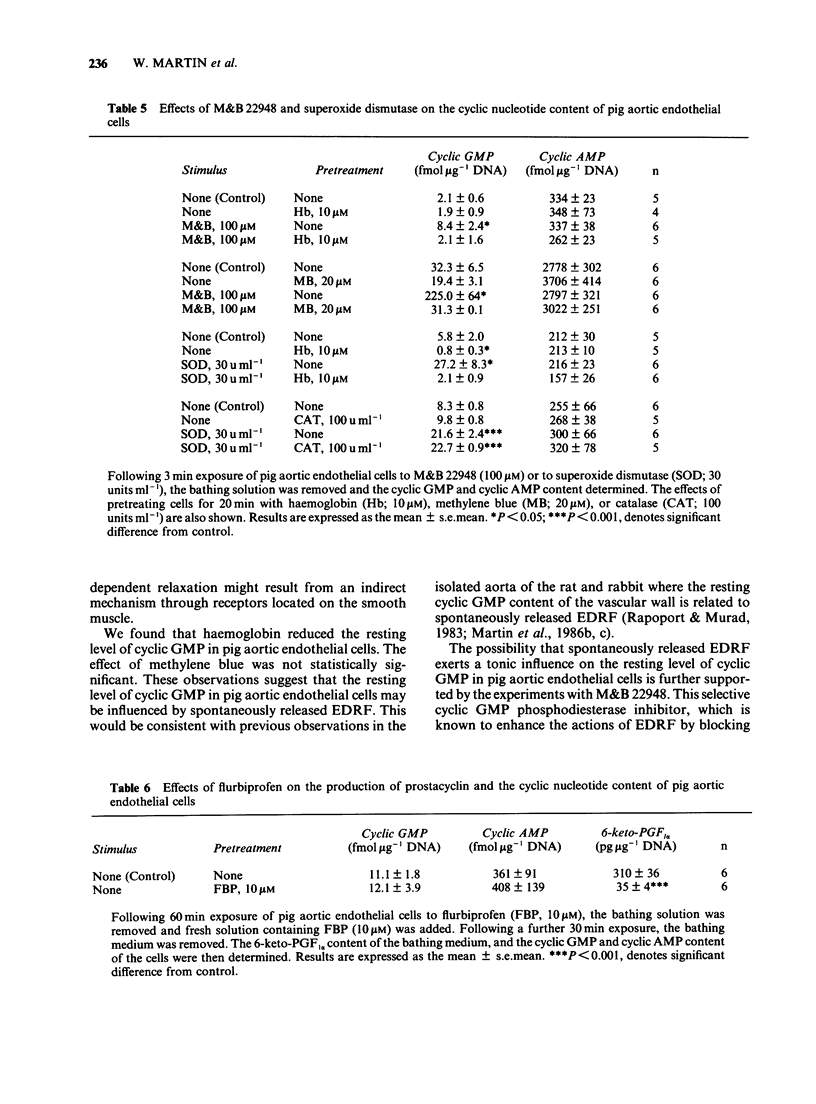
1. Two directly-acting stimulants of soluble guanylate cyclase, glyceryl trinitrate (0.1 microM) and sodium azide (10 microM), and a receptor-mediated stimulant of particulate guanylate cyclase, atriopeptin II (10 nM), each elevated the cyclic GMP content of primary cultures of pig aortic endothelial cells without affecting the cyclic AMP content. 2. Two receptor-mediated stimulants of adenylate cyclase, glucagon (1 microM) and isoprenaline (10 microM), had no effect on the cyclic AMP or cyclic GMP content of these cells, but the directly acting stimulant, forskolin (30 microM), induced a small increase in cyclic AMP content. 3. Three agents that release endothelium-derived relaxing factor (EDRF); bradykinin (0.1 microM), ATP (10 microM) and ionophore A23187 (0.1 microM), each markedly elevated the cyclic GMP content of pig aortic endothelial cells, but acetylcholine (1 microM) had no effect. None of these agents had any effect on cyclic AMP content. 4. Two agents that potentiate the actions of EDRF; M & B 22948 (100 microM) and superoxide dismutase (30 units ml-1), each elevated the cyclic GMP content of pig aortic endothelial cells without affecting the cyclic AMP content. Pretreating cells with catalase (100 units ml-1) did not affect the rise in cyclic GMP content induced by superoxide dismutase (30 units ml-1). 5. Pretreatment of pig aortic endothelial cells with haemoglobin (10 microM) reduced the resting content of cyclic GMP and blocked the increase in cyclic GMP content induced by glyceryl trinitrate (0.1 microM), sodium azide (10 microM), bradykinin (0.1 microM), ATP (10 microM), ionophore A23187 (0.1 microM), M & B 22948 (100 microM) and superoxide dismutase (30 units ml-1), but not that induced by atriopeptin II (10 nM). 6. Pretreatment of pig aortic endothelial cells with an inhibitor of soluble guanylate cyclase, methylene blue (20 microM), had no effect on the resting content of cyclic GMP. Methylene blue (20 microM) blocked the increase in cyclic GMP content induced by glyceryl trinitrate (0.1 microM), M & B22948 (100 microM) and bradykinin (0.1 microM), but not that induced by atriopeptin II (10 nM). 7. The data show that soluble guanylate cyclase, particulate guanylate cyclase and adenylate cyclase are present in pig aortic endothelial cells. They further suggest that EDRF, produced spontaneously or in response to vasoactive agents, elevates endothelial cyclic GMP content by stimulating soluble guanylate cyclase. It is possible that this may serve as a feedback loop by which the endothelial cell modulates EDRF production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Martin W. Loss of receptor-mediated 86Rb efflux from pig aortic endothelial cells in culture. Br J Pharmacol. 1983 Sep;80(1):5–6. doi: 10.1111/j.1476-5381.1983.tb11040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F. Induction of prostacyclin biosynthesis is closely associated with increased guanosine 3',5'-cyclic monophosphate accumulation in cultured human endothelium. J Clin Invest. 1986 Nov;78(5):1253–1260. doi: 10.1172/JCI112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Macfarlane D. E., Hoak J. C. Prostacyclin biosynthesis in vascular endothelium is not inhibited by cyclic AMP. Studies with 3-isobutyl-1-methylxanthine and forskolin. Thromb Res. 1982 Dec 1;28(5):637–647. doi: 10.1016/0049-3848(82)90155-4. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- Currie M. G., Geller D. M., Cole B. R., Boylan J. G., YuSheng W., Holmberg S. W., Needleman P. Bioactive cardiac substances: potent vasorelaxant activity in mammalian atria. Science. 1983 Jul 1;221(4605):71–73. doi: 10.1126/science.6857267. [DOI] [PubMed] [Google Scholar]
- Dembinska-Kiec A., Rücker W., Schönhöfer P. S. PGI2 enhanced cAMP content in bovine coronary arteries in the presence of isobutylmethylxanthine. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):107–110. doi: 10.1007/BF00499051. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ganz P., Davies P. F., Leopold J. A., Gimbrone M. A., Jr, Alexander R. W. Short- and long-term interactions of endothelium and vascular smooth muscle in coculture: effects on cyclic GMP production. Proc Natl Acad Sci U S A. 1986 May;83(10):3552–3556. doi: 10.1073/pnas.83.10.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Stimulation of endothelial prostacyclin production plays no role in endothelium-dependent relaxation of the pig aorta. Br J Pharmacol. 1983 Sep;80(1):179–186. doi: 10.1111/j.1476-5381.1983.tb11064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Henderson A. H. Evidence that cyclic guanosine monophosphate (cGMP) mediates endothelium-dependent relaxation. Eur J Pharmacol. 1985 Jun 7;112(2):195–202. doi: 10.1016/0014-2999(85)90496-0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of endothelial cell cyclic nucleotide metabolism by prostacyclin. J Clin Invest. 1981 Feb;67(2):540–546. doi: 10.1172/JCI110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Krulewitz A. H., Fanburg B. L. Stimulation of bovine endothelial cell angiotensin-I-converting enzyme activity by cyclic AMP-related agents. J Cell Physiol. 1986 Nov;129(2):147–150. doi: 10.1002/jcp.1041290204. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Murad F. Comparison of binding and cyclic GMP accumulation by atrial natriuretic peptides in endothelial cells. Biochim Biophys Acta. 1986 Jan 23;885(1):74–79. doi: 10.1016/0167-4889(86)90040-6. [DOI] [PubMed] [Google Scholar]
- Makarski J. S. Stimulation of cyclic AMP production by vasoactive agents in cultured bovine aortic and pulmonary artery endothelial cells. In Vitro. 1981 May;17(5):450–458. doi: 10.1007/BF02626746. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Morgan R. O., Smith J. A., White D. G. Atriopeptin II-induced relaxation of rabbit aorta is potentiated by M&B 22,948 but not blocked by haemoglobin. Br J Pharmacol. 1986 Nov;89(3):557–561. doi: 10.1111/j.1476-5381.1986.tb11156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Miller O. V., Aiken J. W., Hemker D. P., Shebuski R. J., Gorman R. R. Prostacyclin stimulation of dog arterial cyclic AMP levels. Prostaglandins. 1979 Dec;18(6):915–925. doi: 10.1016/0090-6980(79)90128-x. [DOI] [PubMed] [Google Scholar]
- Mittal C. K. Atriopeptin II and nitrovasodilator-mediated shifts in guanosine 3',5'-cyclic monophosphate in rat thoracic aorta: evidence for involvement of distinct guanylate cyclase pools. Eur J Pharmacol. 1985 Sep 10;115(1):127–128. doi: 10.1016/0014-2999(85)90596-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Murad F., Arnold W. P., Mittal C. K., Braughler J. M. Properties and regulation of guanylate cyclase and some proposed functions for cyclic GMP. Adv Cyclic Nucleotide Res. 1979;11:175–204. [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Singer H. A., Loeb A. L. Mechanisms of endothelium-dependent vascular smooth muscle relaxation. Biochem Pharmacol. 1985 Jun 1;34(11):1867–1874. doi: 10.1016/0006-2952(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A. Prostacyclin release stimulated by thrombin or bradykinin in porcine endothelial cells cultured from aorta and umbilical vein. Thromb Res. 1983 Jan 15;29(2):115–124. doi: 10.1016/0049-3848(83)90133-0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Waldman S. A., Schwartz K., Winquist R. J., Murad F. Effects of atrial natriuretic factor, sodium nitroprusside, and acetylcholine on cyclic GMP levels and relaxation in rat aorta. Eur J Pharmacol. 1985 Sep 24;115(2-3):219–229. doi: 10.1016/0014-2999(85)90694-6. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Stephenson J. A., Summers R. J. Autoradiographic analysis of receptors on vascular endothelium. Eur J Pharmacol. 1987 Jan 28;134(1):35–43. doi: 10.1016/0014-2999(87)90128-2. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. E., Fuller G. C. Dibutyryl cyclic guanosine monophosphate elevates bovine endothelial cell thromboxane production without affecting prostacyclin metabolism. Thromb Res. 1984 Jan 15;33(2):163–175. doi: 10.1016/0049-3848(84)90177-4. [DOI] [PubMed] [Google Scholar]


