Abstract
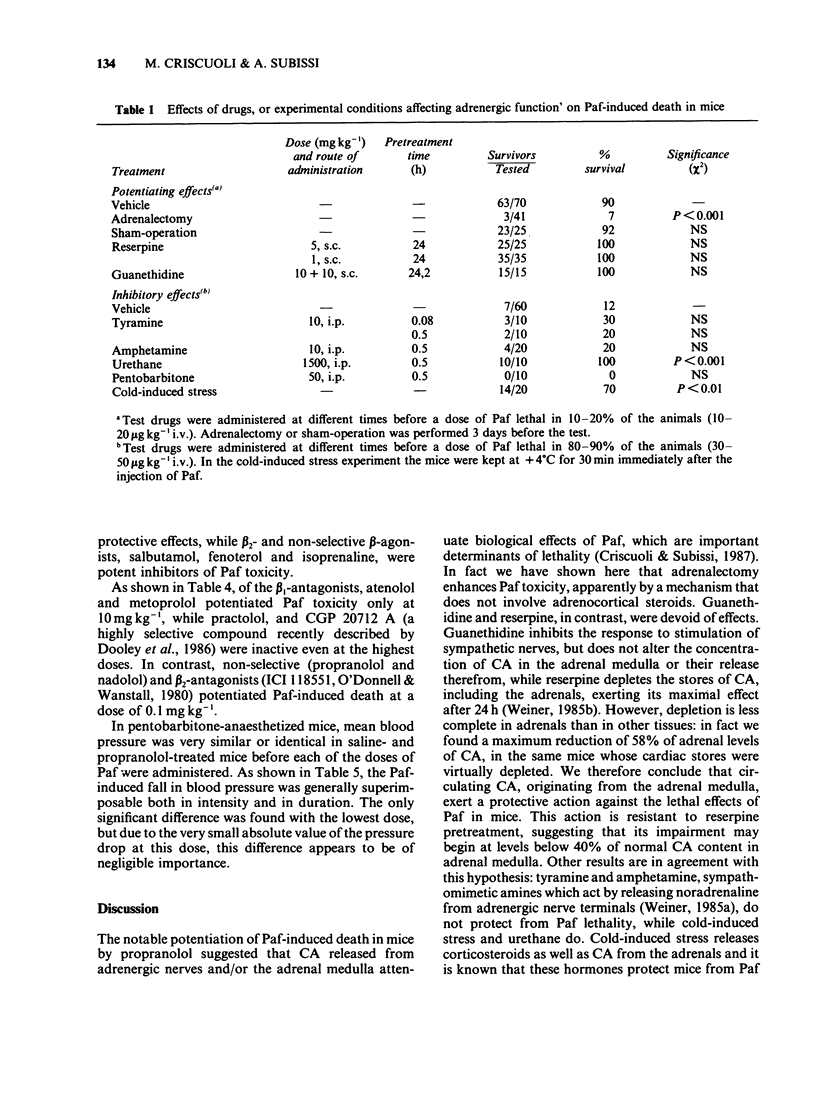
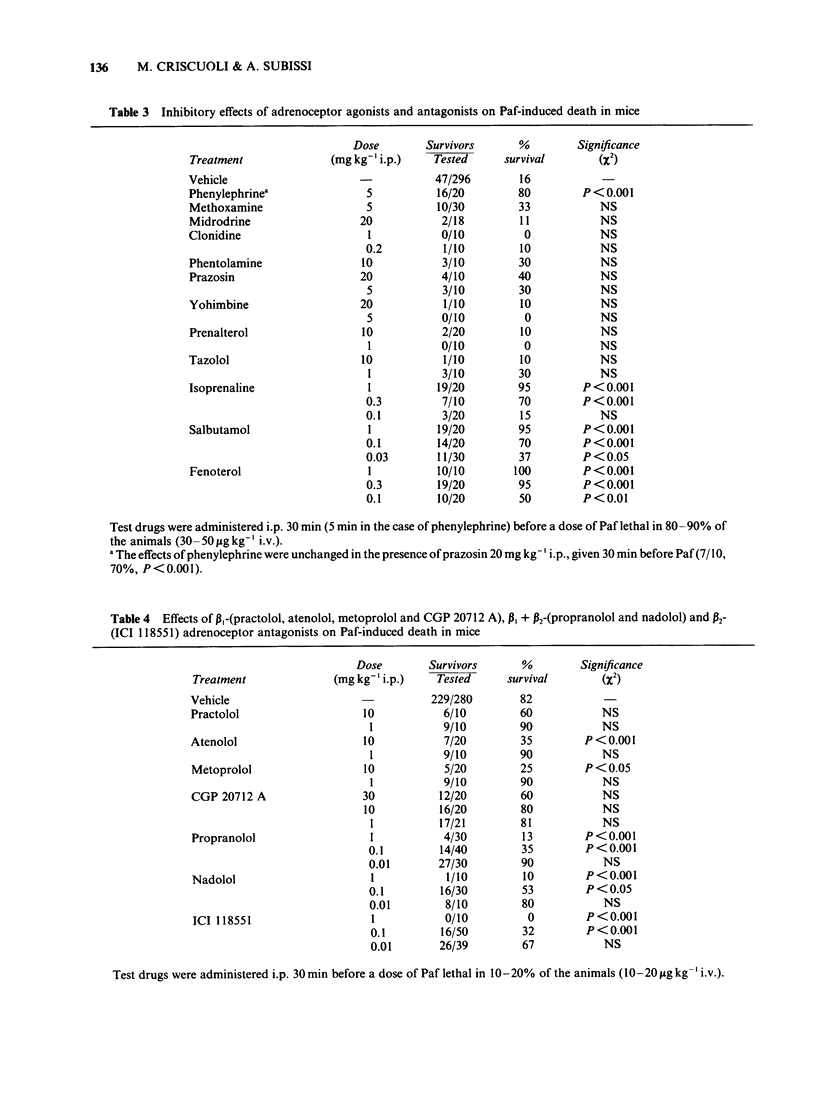
1. The effects of a number of drugs and experimental conditions, which inhibit or stimulate adrenergic function, were evaluated on platelet-activating factor (Paf)-induced death in conscious mice. 2. Adrenalectomy markedly potentiated Paf toxicity, while guanethidine and reserpine did not. However, reserpine, which produced a virtually complete depletion of catecholamines (CA) in cardiac tissue, was not able to reduce adrenal CA by more than 58%. Drugs which release noradrenaline from the adrenergic nerve terminals, such as tyramine and amphetamine, did not protect mice from Paf toxicity, while drugs or conditions which favour the release of CA from the adrenal medulla, such as urethane and cold-induced stress, did. 3. beta 2- and beta 1 + beta 2-adrenoceptor antagonists (ICI 118551, propranolol and nadolol), but not beta 1-antagonists (atenolol, practolol, metoprolol and CGP 20712 A), potentiated Paf toxicity at low doses; beta 2- and beta 1 + beta 2-agonists (salbutamol, fenoterol and isoprenaline), but not beta 1-agonists (prenalterol and tazolol) were potent inhibitors of Paf toxicity. alpha 1- and alpha 2-adrenoceptor agonists and antagonists did not exert significant effects. Propranolol did not appear to enhance the hypotensive action of Paf in pentobarbitone-anaesthetized mice. 4. It is concluded that manipulation of the release of CA from the adrenal medulla, but not from adrenergic nerves, has profound effects on Paf toxicity in mice. A number of considerations support the hypothesis that bronchoconstriction is a major determinant of Paf-induced death in mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Criscuoli M., Subissi A. Paf-acether-induced death in mice: involvement of arachidonate metabolites and beta-adrenoceptors. Br J Pharmacol. 1987 Jan;90(1):203–209. doi: 10.1111/j.1476-5381.1987.tb16841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D. J., Bittiger H., Reymann N. C. CGP 20712 A: a useful tool for quantitating beta 1- and beta 2-adrenoceptors. Eur J Pharmacol. 1986 Oct 14;130(1-2):137–139. doi: 10.1016/0014-2999(86)90193-7. [DOI] [PubMed] [Google Scholar]
- Lefèvre F., Fénard S., Cavero I. Vascular beta-adrenoceptor stimulating properties of phenylephrine. Eur J Pharmacol. 1977 May 1;43(1):85–88. doi: 10.1016/0014-2999(77)90163-7. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986 Feb 15;42(2):109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol. 1979 Jul;16(1):34–46. [PubMed] [Google Scholar]
- Myers A., Ramey E., Ramwell P. Glucocorticoid protection against PAF-acether toxicity in mice. Br J Pharmacol. 1983 Jun;79(2):595–598. doi: 10.1111/j.1476-5381.1983.tb11034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel J. A., Barnes P. J. Autonomic regulation of the airways. Annu Rev Med. 1984;35:451–467. doi: 10.1146/annurev.me.35.020184.002315. [DOI] [PubMed] [Google Scholar]
- Namm D. H., Tadepalli A. S., High J. A. Species specificity of the platelet responses to 1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine. Thromb Res. 1982 Feb 15;25(4):341–350. doi: 10.1016/0049-3848(82)90234-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Evidence that ICI 118, 551 is a potent, highly Beta 2-selective adrenoceptor antagonist and can be used to characterize Beta-adrenoceptor populations in tissues. Life Sci. 1980 Aug 25;27(8):671–677. doi: 10.1016/0024-3205(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Pittner H., Stormann H., Enzenhofer R. Pharmacodynamic actions of midodrine, a new alpha-adrenergic stimulating agent, and its main metabolite, ST 1059. Arzneimittelforschung. 1976;26(12):2145–2154. [PubMed] [Google Scholar]
- Strosberg A. M. The cardiovascular pharmacology and hemodynamic activity of tazolol, a selective myocardial beta-stimulant. Arch Int Pharmacodyn Ther. 1976 Aug;222(2):200–215. [PubMed] [Google Scholar]
- Young J. M., Maloney P. J., Jubb S. N., Clark J. S. Pharmacological investigation of the mechanisms of platelet-activating factor induced mortality in the mouse. Prostaglandins. 1985 Oct;30(4):545–551. doi: 10.1016/0090-6980(85)90018-8. [DOI] [PubMed] [Google Scholar]


