Abstract
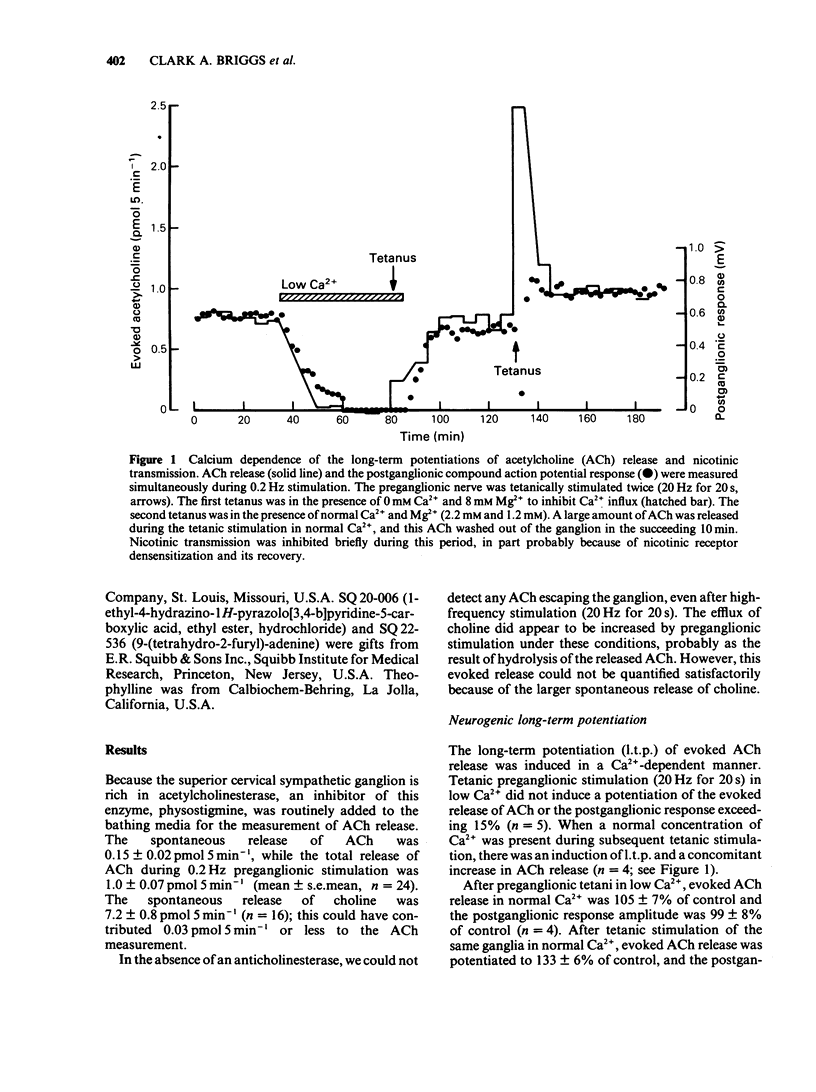
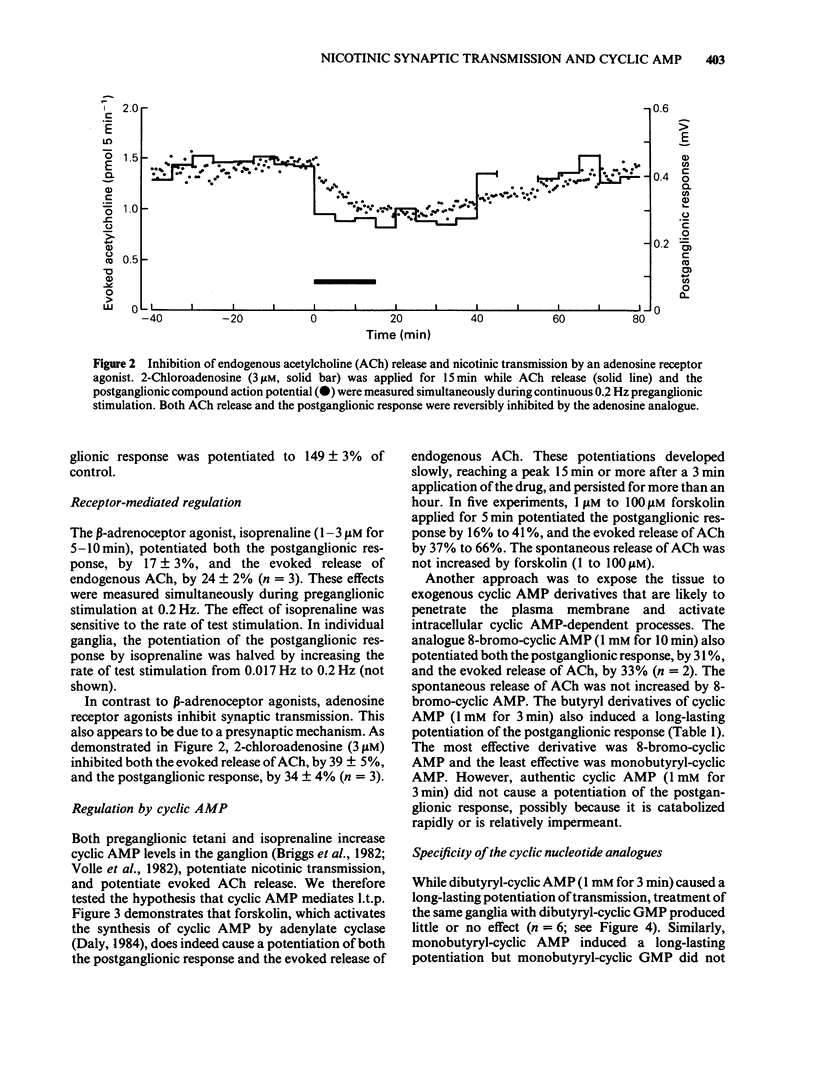
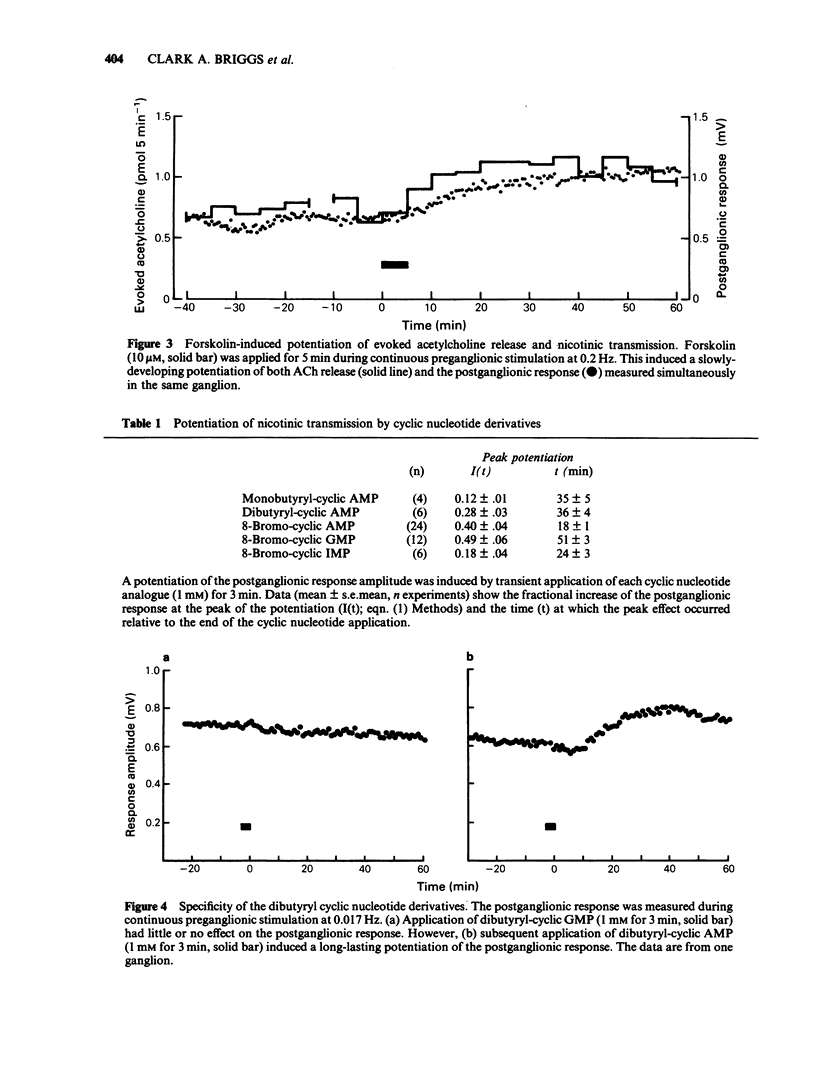
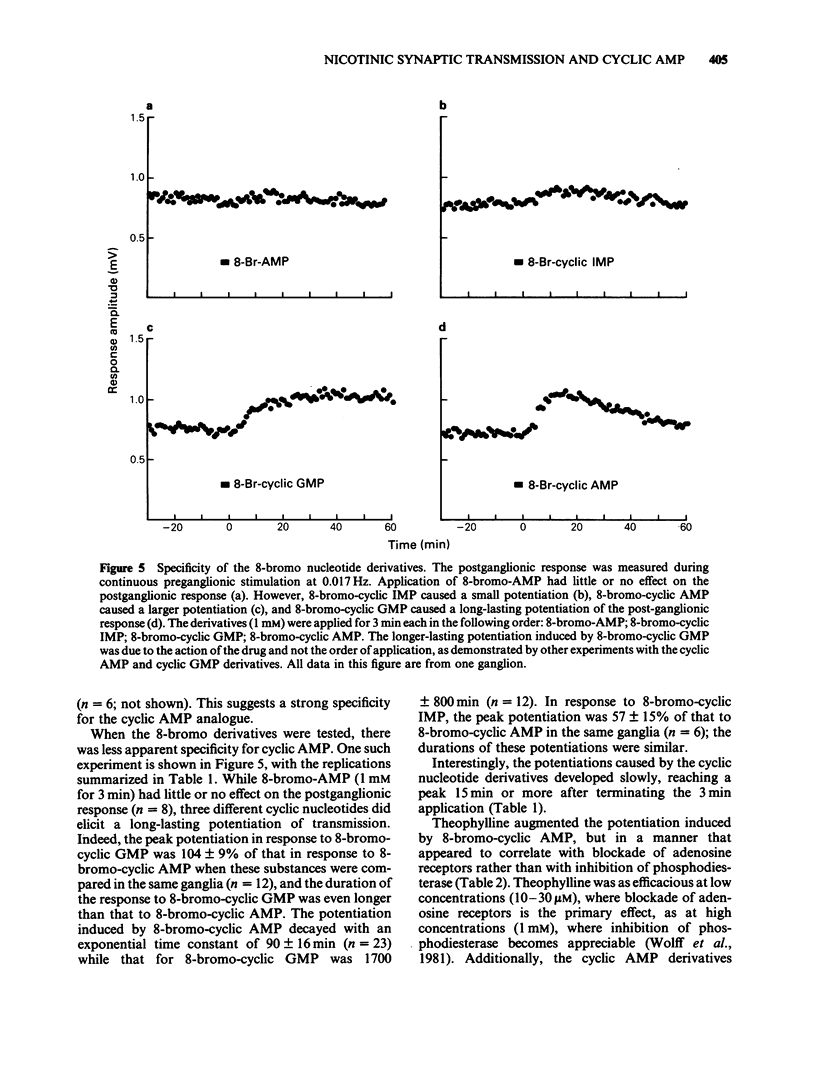
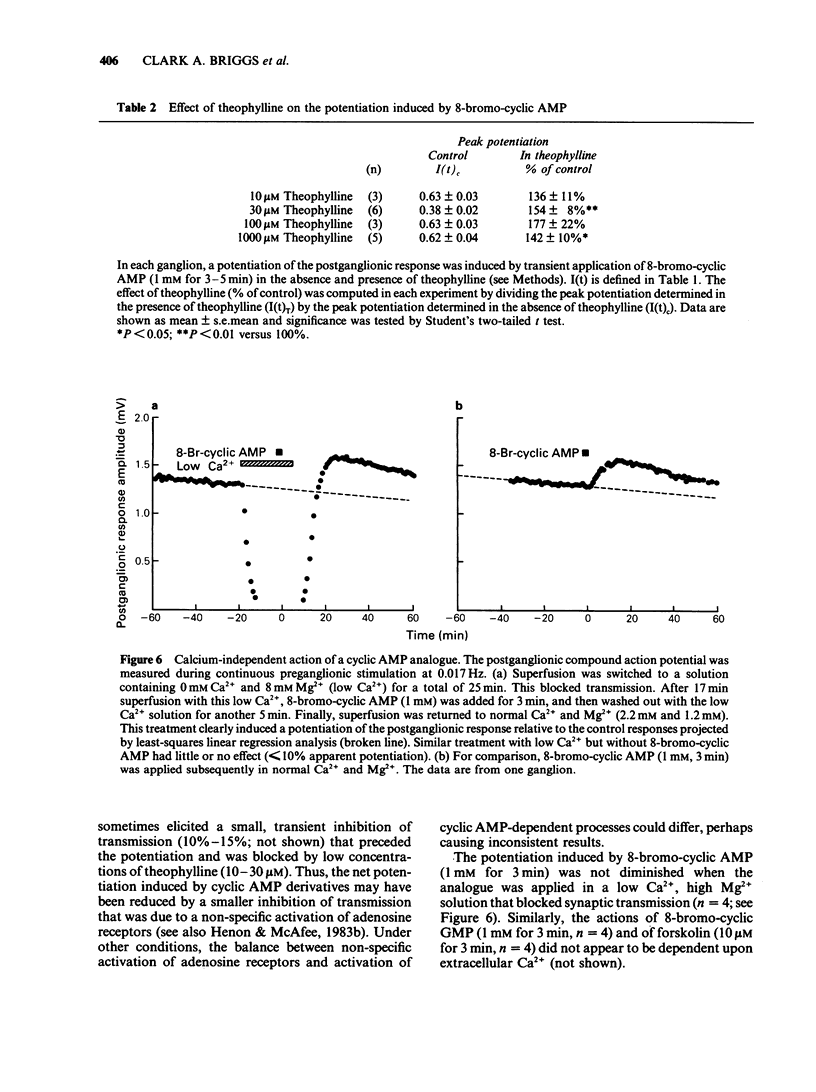
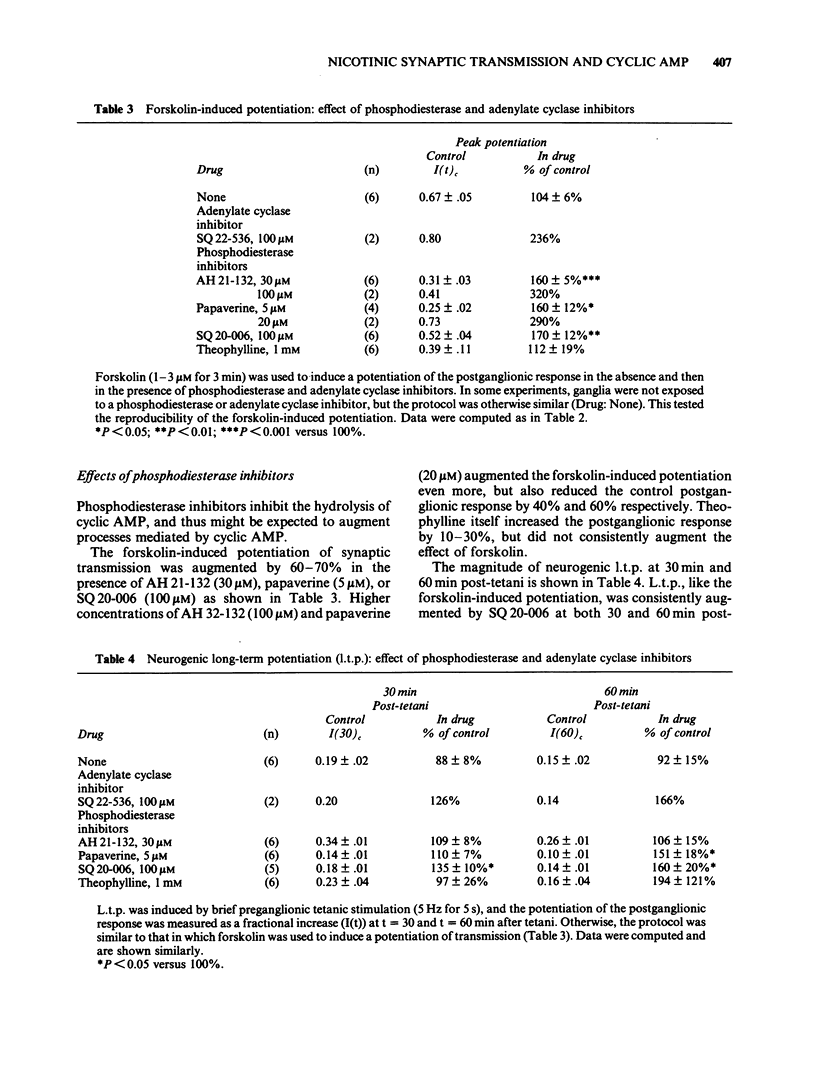
1. Using the rat superior cervical ganglion in vitro, the relative efficacy of nicotinic synaptic transmission was estimated by recording the postganglionic compound action potential and the amount of endogenous acetylcholine (ACh) released. These two parameters were correlated in individual ganglia by sampling the bathing medium for the assay of ACh while simultaneously recording the postganglionic response. 2. The beta-adrenoceptor agonist isoprenaline potentiated both the evoked release of ACh and the postganglionic response by about 20% during preganglionic stimulation at 0.2 Hz. 3. The adenosine receptor agonist 2-chloroadenosine inhibited ACh release and the postganglionic response by about 35%. 4. Tetanic preganglionic stimulation for a few seconds induced a long-term potentiation of nicotinic responses and of ACh release. Both of these potentiations were dependent upon extracellular Ca2+ during the tetani. 5. Forskolin and analogues of cyclic AMP also caused a long-lasting potentiation of both the evoked release of ACh and the postganglionic response, indicating that cyclic AMP may regulate transmission by a presynaptic mechanism. The specificity of the cyclic AMP analogues was tested using various butyryl- and bromo-purine nucleotides. 6. The effects of forskolin and 8-bromo-cyclic AMP did not appear to be dependent upon extracellular Ca2+. 7. The potentiation caused by forskolin was consistently augmented by three phosphodiesterase inhibitors--AH 21-132, papaverine and SQ 20-006. However, the effect of forskolin was not consistently enhanced by theophylline, nor was it reduced by the adenylate cyclase inhibitor SQ 22-536. 8. The neurogenic long-term potentiation was augmented by two of the phosphodiesterase inhibitors that also augmented the forskolin-induced potentiation--papaverine and SQ 20-006. 9. It was concluded that cyclic AMP can enhance nicotinic transmission, and can do so by increasing the evoked release of ACh. However, it was not possible to prove that cyclic AMP mediates the long-term potentiation induced by tetanic preganglionic stimulation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi H., Kudo Y. Opposite actions of forskolin at pre- and postsynaptic sites in rat sympathetic ganglia. Brain Res. 1985 Sep 23;343(2):346–350. doi: 10.1016/0006-8993(85)90753-x. [DOI] [PubMed] [Google Scholar]
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Applegate M. D., Kerr D. S., Landfield P. W. Redistribution of synaptic vesicles during long-term potentiation in the hippocampus. Brain Res. 1987 Jan 20;401(2):401–406. doi: 10.1016/0006-8993(87)91429-6. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Collier B. Evidence that endogenous catecholamines can regulate acetylcholine release in a sympathetic ganglion. Eur J Pharmacol. 1986 Jun 5;125(1):93–101. doi: 10.1016/0014-2999(86)90087-7. [DOI] [PubMed] [Google Scholar]
- Baxter D. A., Bittner G. D., Brown T. H. Quantal mechanism of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5978–5982. doi: 10.1073/pnas.82.17.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985 Jun;363:181–190. doi: 10.1113/jphysiol.1985.sp015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Cyclic adenosine 3',5'-monophosphate and beta-effects in rat isolated superior cervical ganglia. Br J Pharmacol. 1983 Jun;79(2):441–449. doi: 10.1111/j.1476-5381.1983.tb11017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. H. Cellular analysis of associative learning. Physiol Rev. 1987 Apr;67(2):329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:81–89. [PubMed] [Google Scholar]
- DeFrance J. F., Stanley J. C., Marchand J. E., Divakaran P., Clement-Cormier Y. Evidence for a cyclic GMP mechanism in the mediation of hippocampal post-tetanic potentiation. J Neurosci Res. 1983;10(1):35–51. doi: 10.1002/jnr.490100106. [DOI] [PubMed] [Google Scholar]
- Feasey K. J., Lynch M. A., Bliss T. V. Long-term potentiation is associated with an increase in calcium-dependent, potassium-stimulated release of [14C]glutamate from hippocampal slices: an ex vivo study in the rat. Brain Res. 1986 Jan 29;364(1):39–44. doi: 10.1016/0006-8993(86)90985-6. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Henon B. K., McAfee D. A. The ionic basis of adenosine receptor actions on post-ganglionic neurones in the rat. J Physiol. 1983 Mar;336:607–620. doi: 10.1113/jphysiol.1983.sp014600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P., Yamane T. Compartmentalization of cyclic AMP elevation in neurons of Aplysia californica. Cell Mol Neurobiol. 1987 Mar;7(1):19–33. doi: 10.1007/BF00734987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kessler M., Baudry M., Cummins J. T., Way S., Lynch G. Induction of glutamate binding sites in hippocampal membranes by transient exposure to high concentrations of glutamate or glutamate analogs. J Neurosci. 1986 Feb;6(2):355–363. doi: 10.1523/JNEUROSCI.06-02-00355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Kuba K., Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985 Feb;359:219–233. doi: 10.1113/jphysiol.1985.sp015582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Kumamoto E. Long-term potentiation of transmitter release induced by adrenaline in bull-frog sympathetic ganglia. J Physiol. 1986 May;374:515–530. doi: 10.1113/jphysiol.1986.sp016095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch M. A., Errington M. L., Bliss T. V. Long-term potentiation of synaptic transmission in the dentate gyrus: increased release of [14C]glutamate without increase in receptor binding. Neurosci Lett. 1985 Nov 20;62(1):123–129. doi: 10.1016/0304-3940(85)90295-2. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- McCaman M. W., McAfee D. A. Effects of synaptic activity on the metabolism and release of purines in the rat superior cervical ganglion. Cell Mol Neurobiol. 1986 Dec;6(4):349–362. doi: 10.1007/BF00711405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman R. E., Stetzler J. Radiochemical assay for ACh: modifications for sub-picomole measurements. J Neurochem. 1977 Mar;28(3):669–671. doi: 10.1111/j.1471-4159.1977.tb10442.x. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H., Libet B. Stimulation of adenylate cyclase in relation to dopamine-induced long-term enhancement (LTE) of muscarinic depolarization in the rabbit superior cervical ganglion. J Neurosci. 1987 Feb;7(2):311–318. doi: 10.1523/JNEUROSCI.07-02-00311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Walaas S. I., Greengard P. Neuronal phosphoproteins: physiological and clinical implications. Science. 1984 Sep 21;225(4668):1357–1364. doi: 10.1126/science.6474180. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Barraco R. A. Adenosine, adenylate cyclase, and transmitter release. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:243–257. [PubMed] [Google Scholar]
- Quenzer L. F., Patterson B. A., Volle R. L. The cyclic nucleotide content of the rat superior cervical ganglion. J Pharmacol Exp Ther. 1980 Nov;215(2):297–303. [PubMed] [Google Scholar]
- Reese J. H., Cooper J. R. Stimulation of acetylcholine release from guinea-pig ileal synaptosomes by cyclic nucleotides and forskolin. Biochem Pharmacol. 1984 Oct 1;33(19):3007–3011. doi: 10.1016/0006-2952(84)90601-4. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985 Aug;5(8):2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res. 1985 Dec 9;358(1-2):343–348. doi: 10.1016/0006-8993(85)90981-3. [DOI] [PubMed] [Google Scholar]
- Volle R. L., Quenzer L. F., Patterson B. A. The regulation of cyclic nucleotides in a sympathetic ganglion. J Auton Nerv Syst. 1982 Jul;6(1):65–72. doi: 10.1016/0165-1838(82)90023-6. [DOI] [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]


