Abstract
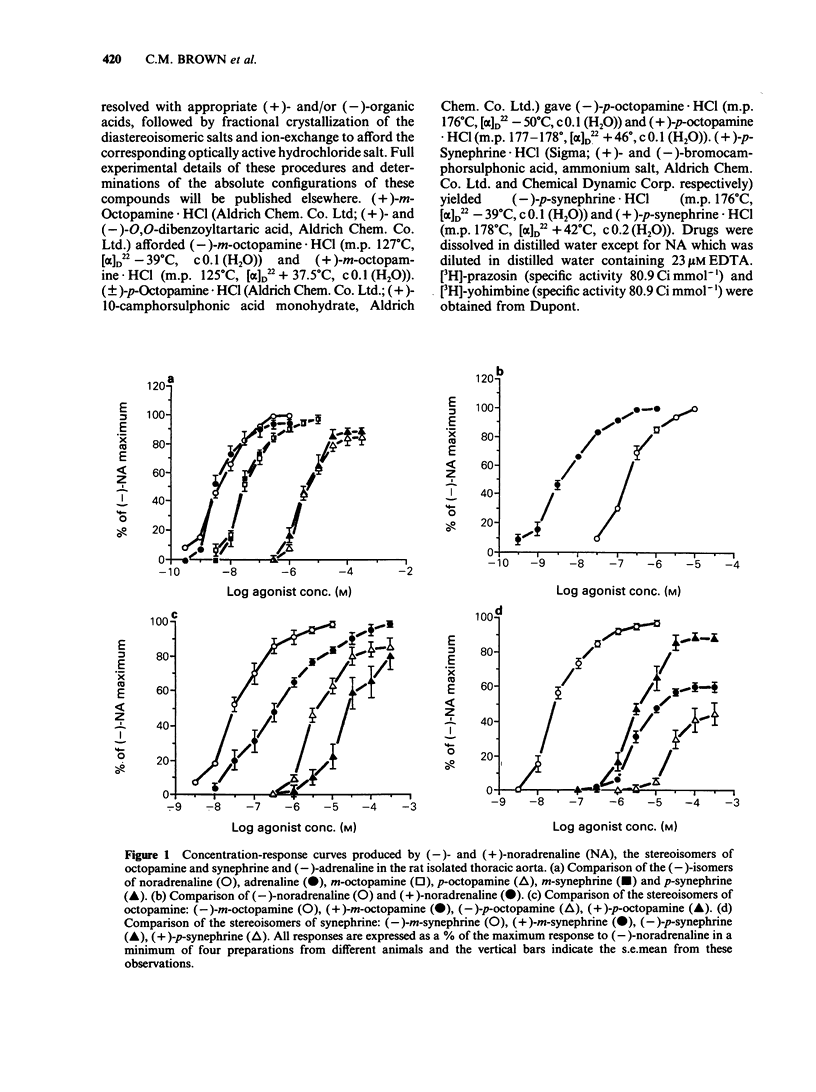
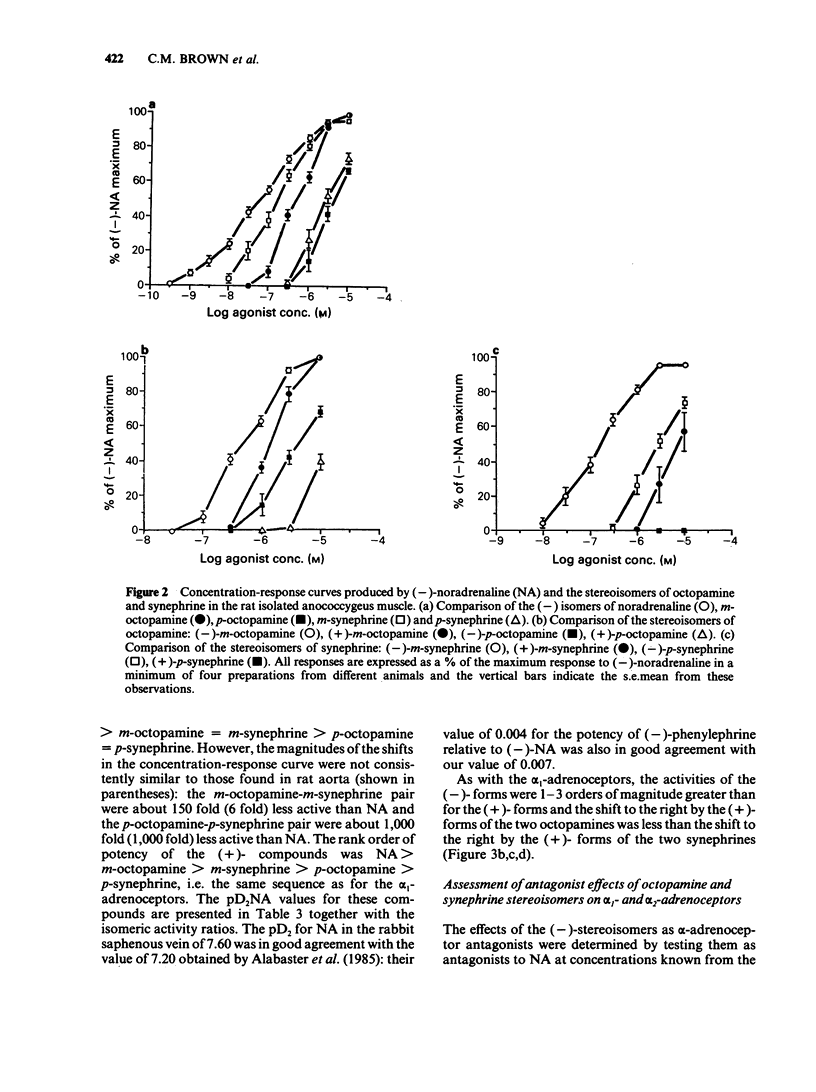
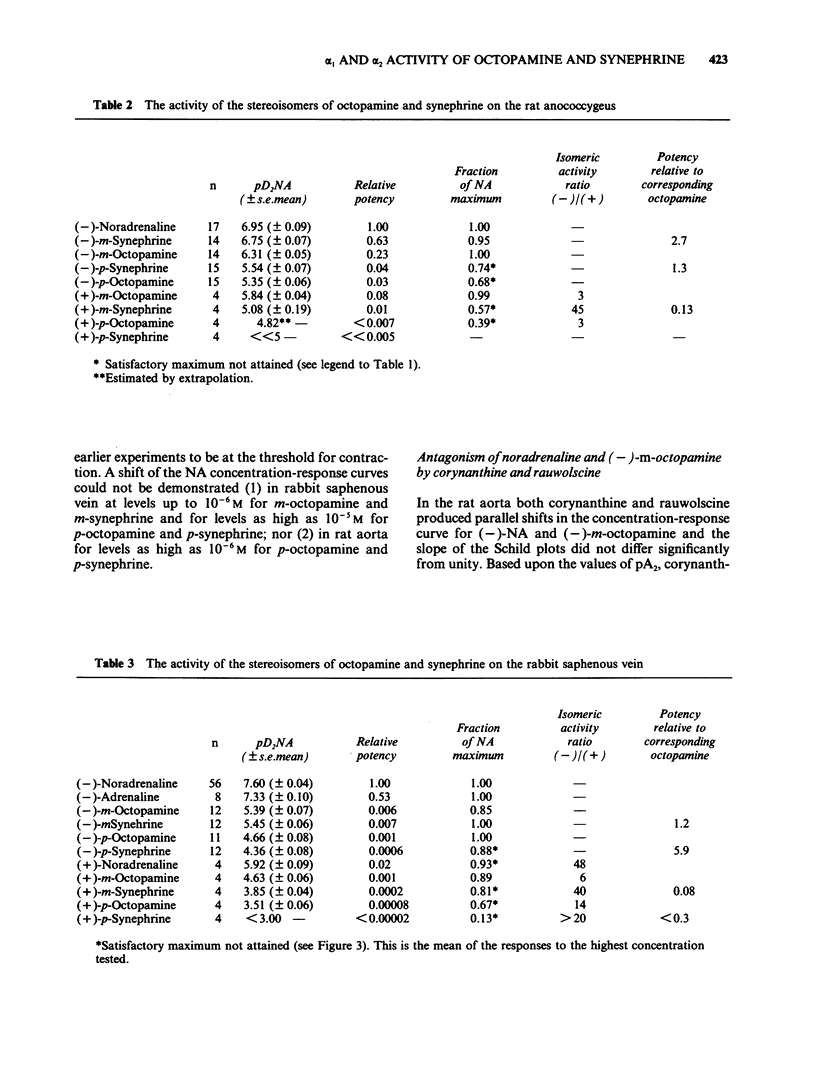
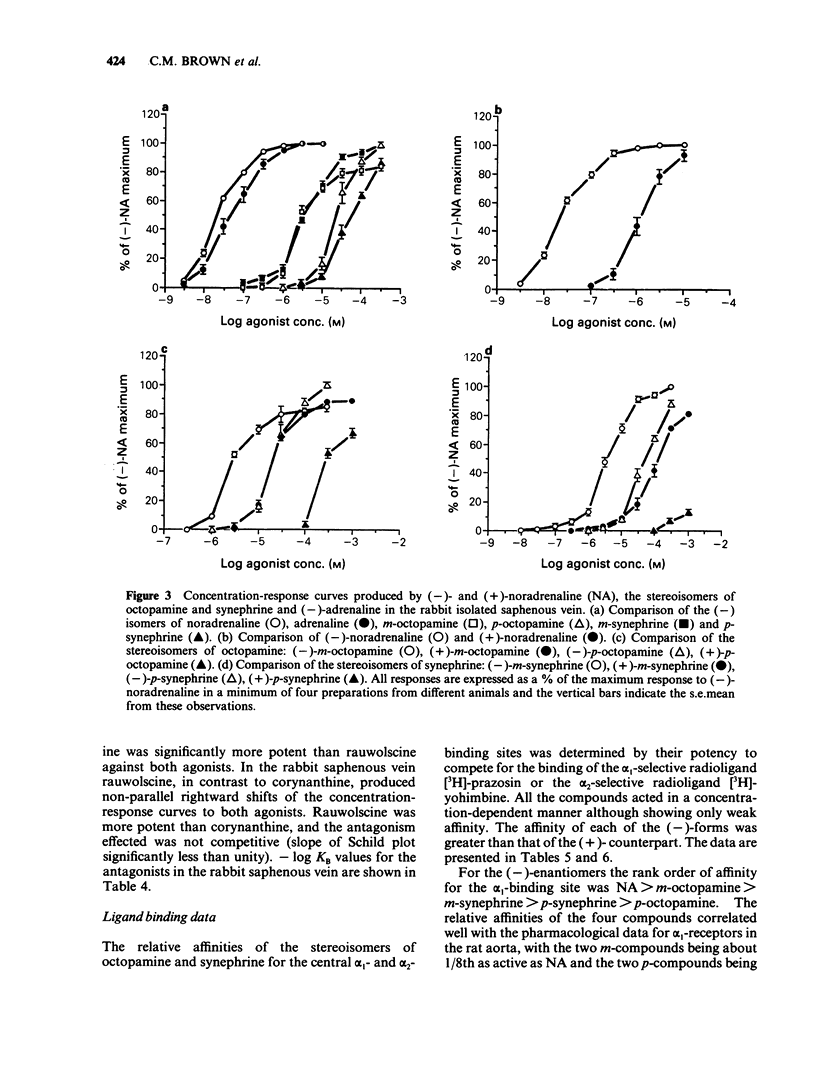
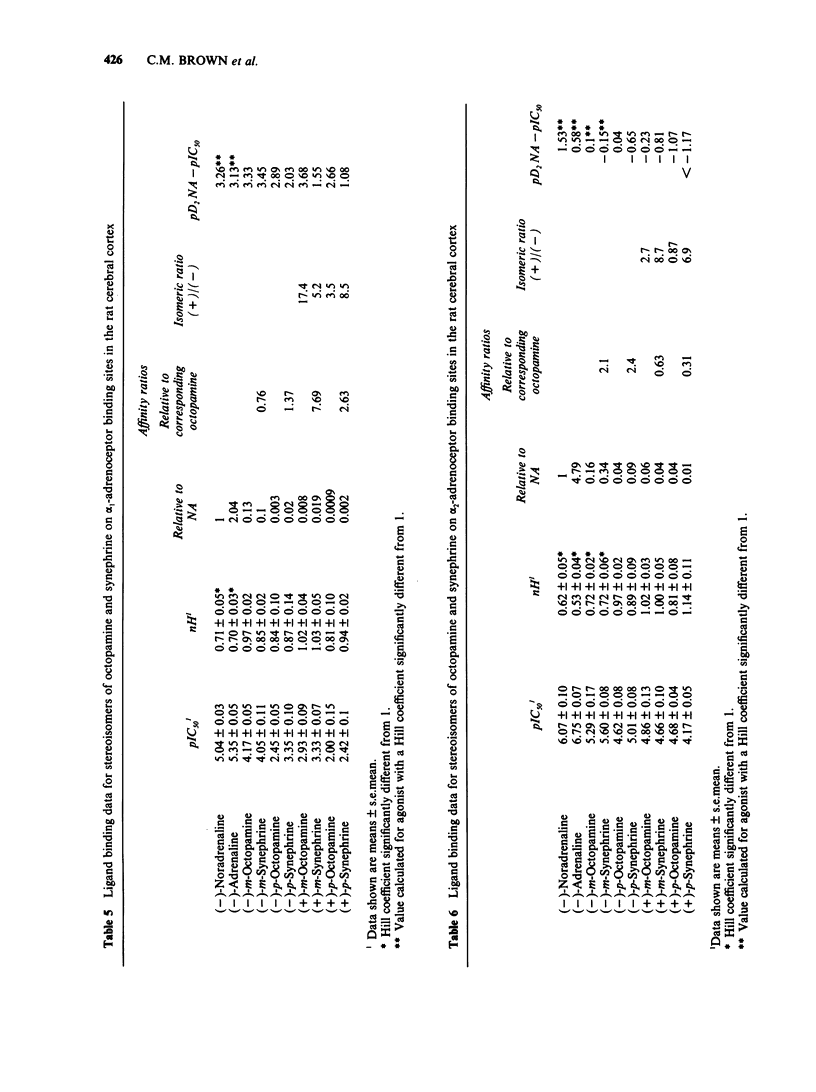
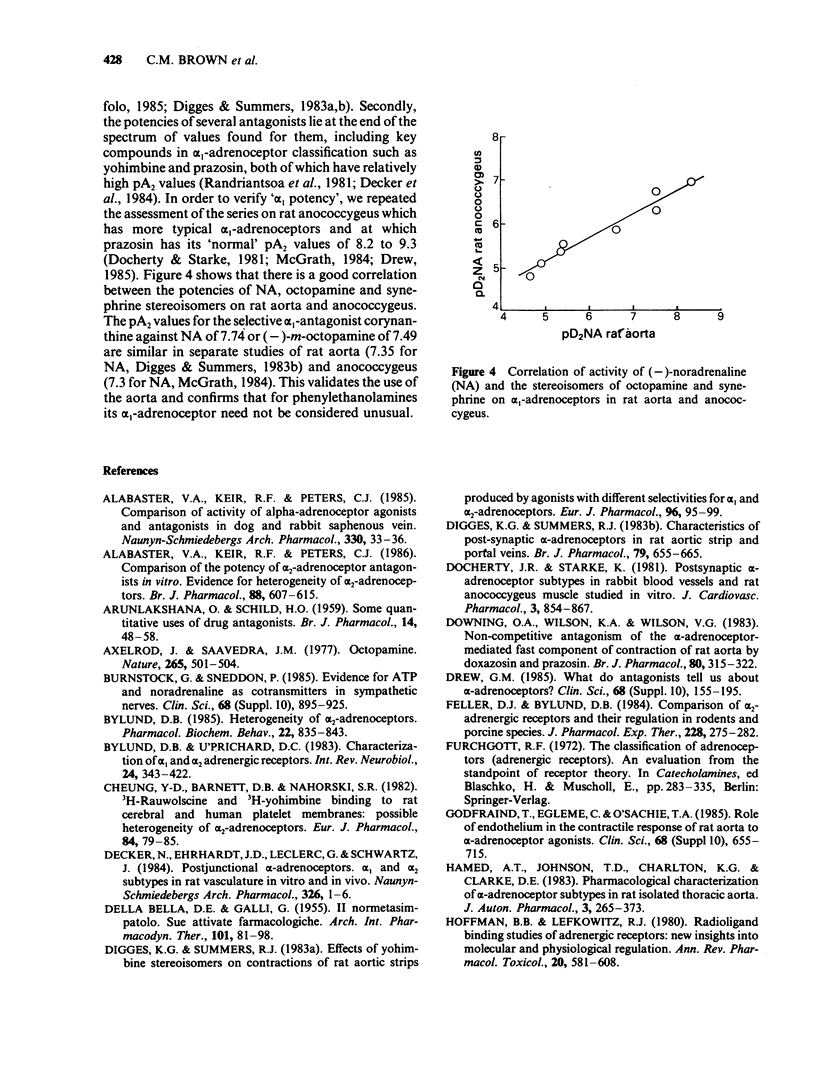
1. The activities of the (-)- and (+)-forms of m- and p-octopamine and m- and p-synephrine on alpha 1-adrenoceptors from rat aorta and anococcygeus and alpha 2-adrenoceptors from rabbit saphenous vein were compared with those of noradrenaline (NA). 2. The rank order of potency of the (-)-forms on alpha 1-adrenoceptors from rat aorta and alpha 2-adrenoceptors was NA greater than m-octopamine = m-synephrine greater than p-octopamine = p-synephrine. The two m-compounds were 6 fold less active than NA on alpha 1-adrenoceptors from rat aorta and 150 fold less active on alpha 2-adrenoceptors. The two p- compounds were 1,000 fold less active than NA on both alpha 1-adrenoceptors from rat aorta and alpha 2-adrenoceptors. The rank order of potency of the (-)- forms on alpha 1-adrenoceptors from rat anococcygeus was NA = m-synephrine greater than m-octopamine greater than p-octopamine = p-synephrine. m-Octopamine was 4 fold less active than NA and (-)-m-synephrine. The two p- compounds were 30 fold less active than NA. 3. The rank order of potency of the (+)- forms was NA greater than m-octopamine greater than m-synephrine greater than p-octopamine greater than p-synephrine on both alpha 1- and alpha 2-adrenoceptors. The potency of each (+)- form was 1-2 orders of magnitude less than that of the (-) counterpart, the differences being greater for the stereoisomers of synephrine than for those of octopamine on both alpha 1- and alpha 2-adrenoceptors. 4. The yohimbine diastereoisomer antagonists, rauwolscine and corynanthine, were tested against (-)-NA and (-)-m-octopamine-induced contractions in both preparations. Based upon the known selectivities of these isomers for alpha-adrenoceptor subtypes, it is concluded that the rat aorta contains only alpha 1-adrenoceptors while the rabbit saphenous vein possesses predominantly alpha 2-adrenoceptors. 5. Ligand binding data for the octopamine and synephrine stereoisomers at alpha 1- and alpha 2-binding sites from rat cerebral cortex was also obtained. (-)-Forms were more active than (+)-forms. The rank order of affinity of the (-)-forms for both alpha 1- and alpha 2-binding sites was NA greater than m-octopamine = m-synephrine greater than p-synephrine greater than p-octopamine. The relative affinities of the members of the series against alpha 1-binding sites were very similar to their relative functional activities on rat aorta.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabaster V. A., Keir R. F., Peters C. J. Comparison of activity of alpha-adrenoceptor agonists and antagonists in dog and rabbit saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jul;330(1):33–36. doi: 10.1007/BF00586706. [DOI] [PubMed] [Google Scholar]
- Alabaster V. A., Keir R. F., Peters C. J. Comparison of potency of alpha 2-adrenoceptor antagonists in vitro: evidence for heterogeneity of alpha 2-adrenoceptors. Br J Pharmacol. 1986 Jul;88(3):607–614. doi: 10.1111/j.1476-5381.1986.tb10241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J., Saavedra J. M. Octopamine. Nature. 1977 Feb 10;265(5594):501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Bylund D. B. Heterogeneity of alpha-2 adrenergic receptors. Pharmacol Biochem Behav. 1985 May;22(5):835–843. doi: 10.1016/0091-3057(85)90536-2. [DOI] [PubMed] [Google Scholar]
- Bylund D. B., U'Prichard D. C. Characterization of alpha 1- and alpha 2-adrenergic receptors. Int Rev Neurobiol. 1983;24:343–431. [PubMed] [Google Scholar]
- Cheung Y. D., Barnett D. B., Nahorski S. R. [3H]Rauwolscine and [3H]yohimbine binding to rat cerebral and human platelet membranes: possible heterogeneity of alpha 2-adrenoceptors. Eur J Pharmacol. 1982 Oct 15;84(1-2):79–85. doi: 10.1016/0014-2999(82)90159-5. [DOI] [PubMed] [Google Scholar]
- DELLA BELLA D., GALLI G. Il normetasimpatolo: sue attività farmacologiche. Arch Int Pharmacodyn Ther. 1955 Feb 1;101(1):81–98. [PubMed] [Google Scholar]
- Decker N., Ehrhardt J. D., Leclerc G., Schwartz J. Postjunctional alpha-adrenoceptors. Alpha 1 and alpha 2 subtypes in rat vasculature in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1984 May;326(1):1–6. doi: 10.1007/BF00518771. [DOI] [PubMed] [Google Scholar]
- Digges K. G., Summers R. J. Characterization of postsynaptic alpha-adrenoceptors in rat aortic strips and portal veins. Br J Pharmacol. 1983 Jul;79(3):655–665. doi: 10.1111/j.1476-5381.1983.tb10002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digges K. G., Summers R. J. Effects of yohimbine stereoisomers on contractions of rat aortic strips produced by agonists with different selectivity for alpha 1- and alpha 2-adrenoceptors. Eur J Pharmacol. 1983 Dec 9;96(1-2):95–99. doi: 10.1016/0014-2999(83)90533-2. [DOI] [PubMed] [Google Scholar]
- Docherty J. R., Starke K. Postsynaptic alpha-adrenoceptor subtypes in rabbit blood vessels and rat anococcygeus muscle studied in vitro. J Cardiovasc Pharmacol. 1981 Jul-Aug;3(4):854–866. doi: 10.1097/00005344-198107000-00019. [DOI] [PubMed] [Google Scholar]
- Downing O. A., Wilson K. A., Wilson V. G. Non-competitive antagonism of the alpha-adrenoceptor-mediated fast component of contraction of rat aorta, by doxazosin and prazosin. Br J Pharmacol. 1983 Oct;80(2):315–322. doi: 10.1111/j.1476-5381.1983.tb10036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller D. J., Bylund D. B. Comparison of alpha-2 adrenergic receptors and their regulation in rodent and porcine species. J Pharmacol Exp Ther. 1984 Feb;228(2):275–282. [PubMed] [Google Scholar]
- Hamed A. T., Johnson T. D., Charlton K. G., Clarke D. E. Pharmacological characterization of alpha-adrenoreceptor subtypes in rat isolated thoracic aorta. J Auton Pharmacol. 1983 Dec;3(4):265–273. doi: 10.1111/j.1474-8673.1983.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Lefkowitz R. J. Radioligand binding studies of adrenergic receptors: new insights into molecular and physiological regulation. Annu Rev Pharmacol Toxicol. 1980;20:581–608. doi: 10.1146/annurev.pa.20.040180.003053. [DOI] [PubMed] [Google Scholar]
- Ibrahim K. E., Couch M. W., Williams C. M., Fregly M. J., Midgley J. M. m-Octopamine: normal occurrence with p-octopamine in mammalian sympathetic nerves. J Neurochem. 1985 Jun;44(6):1862–1867. doi: 10.1111/j.1471-4159.1985.tb07180.x. [DOI] [PubMed] [Google Scholar]
- Jordan R., Midgley J. M., Thonoor C. M., Williams C. M. Beta-adrenergic activities of octopamine and synephrine stereoisomers on guinea-pig atria and trachea. J Pharm Pharmacol. 1987 Sep;39(9):752–754. doi: 10.1111/j.2042-7158.1987.tb06986.x. [DOI] [PubMed] [Google Scholar]
- KOPIN I. J., FISCHER J. E., MUSACCHIO J., HORST W. D. EVIDENCE FOR A FALSE NEUROCHEMICAL TRANSMITTER AS A MECHANISM FOR THE HYPOTENSIVE EFFECT OF MONOAMINE OXIDASE INHIBITORS. Proc Natl Acad Sci U S A. 1964 Sep;52:716–721. doi: 10.1073/pnas.52.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol B., Soffer L., Brown M. L. Some cardiovascular studies on octopamine. Arch Int Pharmacodyn Ther. 1968 Feb;171(2):415–424. [PubMed] [Google Scholar]
- LANDS A. M., GRANT J. I. The vasopressor action and toxicity of cyclohexylethylamine derivatives. J Pharmacol Exp Ther. 1952 Nov;106(3):341–345. [PubMed] [Google Scholar]
- LANDS A. M. The cardiovascular actions 1-(3-aminophenyl)-2-aminoethanol and related compounds. J Pharmacol Exp Ther. 1952 Apr;104(4):474–477. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latifpour J., Jones S. B., Bylund D. B. Characterization of [3H]yohimbine binding to putative alpha-2 adrenergic receptors in neonatal rat lung. J Pharmacol Exp Ther. 1982 Dec;223(3):606–611. [PubMed] [Google Scholar]
- McGrath J. C. Alpha-adrenoceptor antagonism by apoyohimbine and some observations on the pharmacology of alpha-adrenoceptors in the rat anococcygeus and vas deferens. Br J Pharmacol. 1984 Aug;82(4):769–781. doi: 10.1111/j.1476-5381.1984.tb16473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. C. Evidence for more than one type of post-junctional alpha-adrenoceptor. Biochem Pharmacol. 1982 Feb 15;31(4):467–484. doi: 10.1016/0006-2952(82)90147-2. [DOI] [PubMed] [Google Scholar]
- Randriantsoa A., Heitz C., Stoclet J. C. Functional characterization of postjunctional alpha-adrenoceptors in rat aorta. Eur J Pharmacol. 1981 Oct 15;75(1):57–60. doi: 10.1016/0014-2999(81)90345-9. [DOI] [PubMed] [Google Scholar]
- Reimann W. Evidence for neuronal uptake and release of norfenefrine and evaluation of its alpha-agonistic effects in the rabbit pulmonary artery. Arzneimittelforschung. 1984;34(5):575–578. [PubMed] [Google Scholar]
- Ress R. J., Rahmani M. A., Fregly M. J., Field F. P., Williams C. M. Effect of isomers of octopamine on in vitro reactivity of vascular smooth muscle of rats. Pharmacology. 1980;21(5):342–347. doi: 10.1159/000137450. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr Relative agonist potency as a means of differentiating alpha-adrenoceptors and alpha-adrenergic mechanisms. Clin Sci (Lond) 1985;68 (Suppl 10):9s–14s. doi: 10.1042/cs068s009. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Waddell J. E. Aromatic and benzylic hydroxyl substitution of imidazolines and phenethylamines: differences in activity at alpha-1 and alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1983 Mar;224(3):559–566. [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Yaden E. L., Waddell J. E. Stereochemical requirements of alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1982 Sep;222(3):645–651. [PubMed] [Google Scholar]



