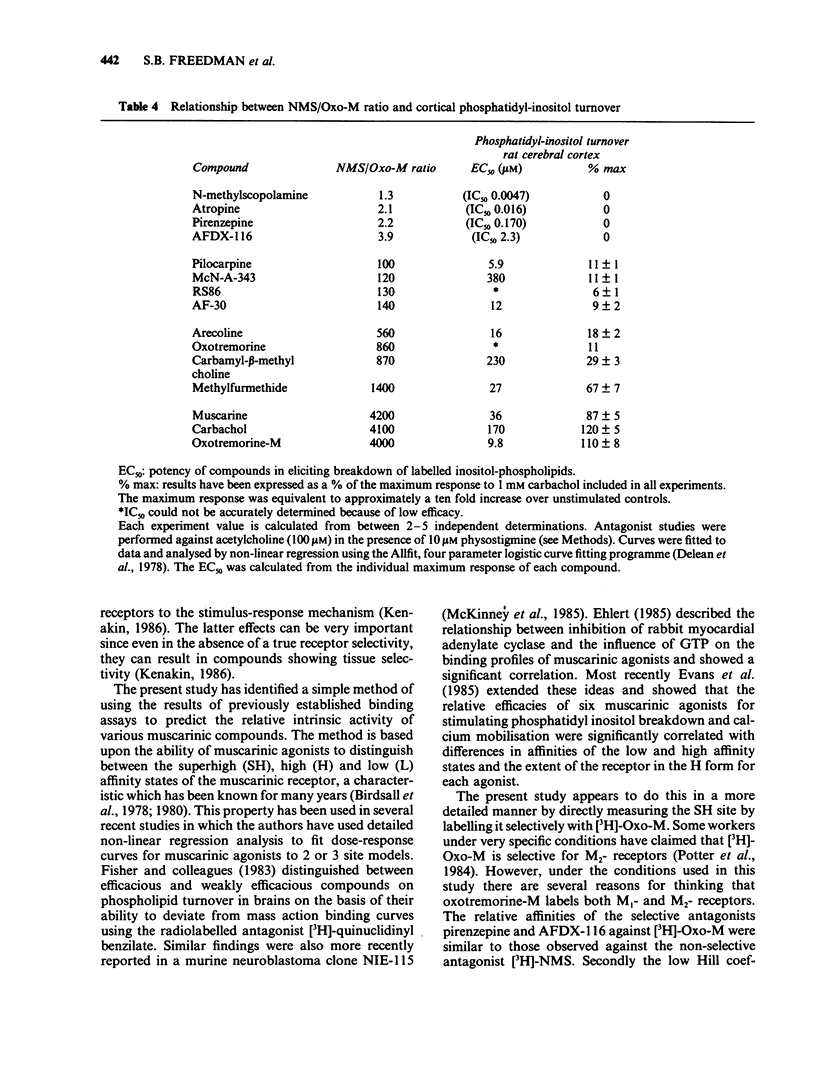
Abstract
1. Radioligand binding assays using [3H]-N-methylscopolamine (NMS) and [3H]-oxotremorine M (Oxo-M) have been devised to predict the efficacy of test compounds at muscarinic receptors in rat cerebral cortex. 2. Muscarinic antagonists, including non-selective and both M1- and M2-selective compounds, displayed similar affinity for both binding assays. 3. Full agonists such as carbachol and muscarine possessed a ratio of potencies against the antagonist versus the agonist ligand (NMS/Oxo-M ratio) of greater than 4000. 4. Compounds which have been shown previously to display partial agonist activity in functional assays e.g. pilocarpine and RS86 had intermediate NMS/Oxo-M ratios of 100-150. A second group of compounds which included oxotremorine had somewhat higher ratios (500-1400). 5. The ratio of affinity constants for the two assays predicted the ability of agonists to stimulate cortical phosphatidyl-inositol turnover. 6. These results suggest that the NMS/Oxo-M ratio may be a useful prediction of efficacy for novel compounds acting at cortical muscarinic receptors.
Full text
PDF






Selected References
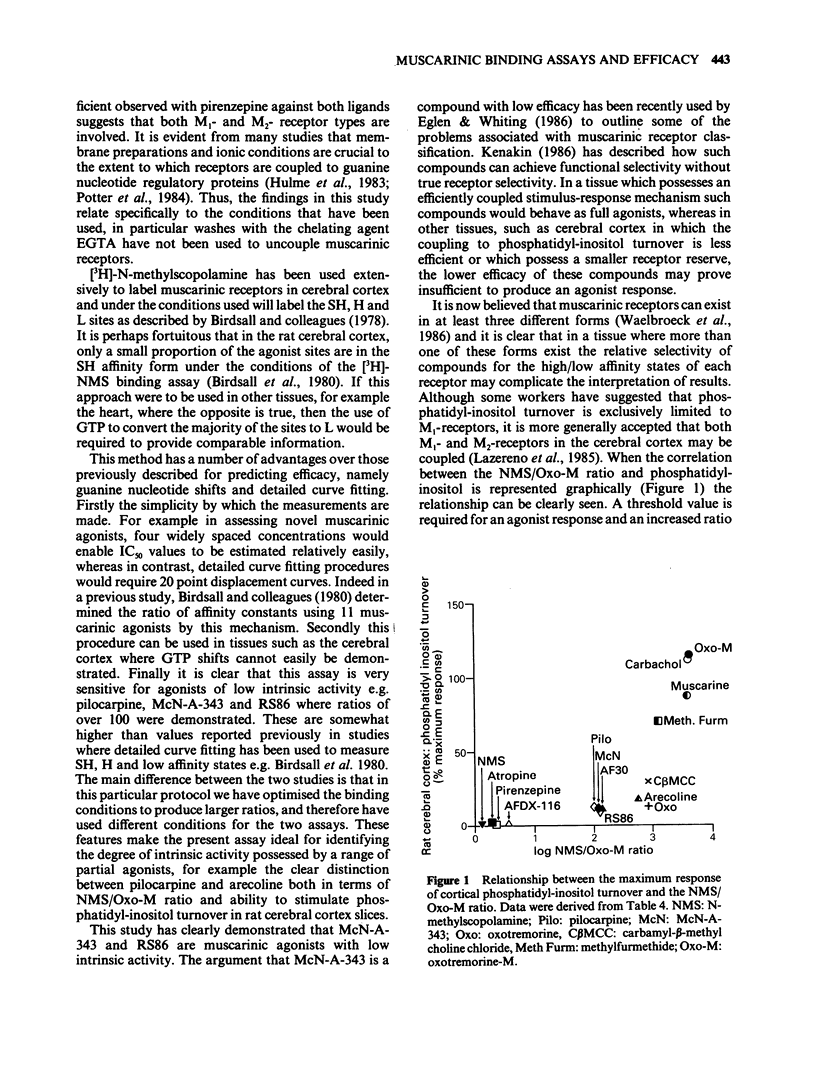
These references are in PubMed. This may not be the complete list of references from this article.
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- Birdsall N. J., Hulme E. C., Burgen A. The character of the muscarinic receptors in different regions of the rat brain. Proc R Soc Lond B Biol Sci. 1980 Feb 13;207(1166):1–12. doi: 10.1098/rspb.1980.0011. [DOI] [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dusting G. J., Li D. M. Catecholamine release and potentiation of thromboxane A2 production by nicotine in the greyhound. Br J Pharmacol. 1986 Jan;87(1):29–36. doi: 10.1111/j.1476-5381.1986.tb10153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Ehlert F. J. The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol Pharmacol. 1985 Nov;28(5):410–421. [PubMed] [Google Scholar]
- Evans T., Hepler J. R., Masters S. B., Brown J. H., Harden T. K. Guanine nucleotide regulation of agonist binding to muscarinic cholinergic receptors. Relation to efficacy of agonists for stimulation of phosphoinositide breakdown and Ca2+ mobilization. Biochem J. 1985 Dec 15;232(3):751–757. doi: 10.1042/bj2320751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A., Weinstock M., Gitter S., Cohen S. A new probe for heterogeneity in muscarinic receptors: 2-methyl-spiro-(1, 3-dioxolane-4, 3')-quinuclidine. Eur J Pharmacol. 1976 Jun;37(2):329–338. doi: 10.1016/0014-2999(76)90041-8. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Bartus R. T. Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem. 1985 Oct;45(4):1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Figueiredo J. C., Bartus R. T. Differential stimulation of inositol phospholipid turnover in brain by analogs of oxotremorine. J Neurochem. 1984 Oct;43(4):1171–1179. doi: 10.1111/j.1471-4159.1984.tb12858.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Klinger P. D., Agranoff B. W. Muscarinic agonist binding and phospholipid turnover in brain. J Biol Chem. 1983 Jun 25;258(12):7358–7363. [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Berrie C. P., Birdsall N. J., Jameson M., Stockton J. M. Regulation of muscarinic agonist binding by cations and guanine nucleotides. Eur J Pharmacol. 1983 Oct 14;94(1-2):59–72. doi: 10.1016/0014-2999(83)90442-9. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Burgen A. S., Mehta P. The binding of antagonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):737–750. [PubMed] [Google Scholar]
- Jacobson M. D., Wusteman M., Downes C. P. Muscarinic receptors and hydrolysis of inositol phospholipids in rat cerebral cortex and parotid gland. J Neurochem. 1985 Feb;44(2):465–472. doi: 10.1111/j.1471-4159.1985.tb05437.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazareno S., Kendall D. A., Nahorski S. R. Pirenzepine indicates heterogeneity of muscarinic receptors linked to cerebral inositol phospholipid metabolism. Neuropharmacology. 1985 Jun;24(6):593–595. doi: 10.1016/0028-3908(85)90071-1. [DOI] [PubMed] [Google Scholar]
- McKinney M., Stenstrom S., Richelson E. Muscarinic responses and binding in a murine neuroblastoma clone (N1E-115). Mediation of separate responses by high affinity and low affinity agonist-receptor conformations. Mol Pharmacol. 1985 Feb;27(2):223–235. [PubMed] [Google Scholar]
- Olianas M. C., Onali P., Neff N. H., Costa E. Adenylate cyclase activity of synaptic membranes from rat striatum. Inhibition by muscarinic receptor agonists. Mol Pharmacol. 1983 Mar;23(2):393–398. [PubMed] [Google Scholar]
- Palacios J. M., Bolliger G., Closse A., Enz A., Gmelin G., Malanowski J. The pharmacological assessment of RS 86 (2-ethyl-8-methyl-2,8-diazaspiro-[4,5]-decan-1,3-dion hydrobromide). A potent, specific muscarinic acetylcholine receptor agonist. Eur J Pharmacol. 1986 Jun 5;125(1):45–62. doi: 10.1016/0014-2999(86)90082-8. [DOI] [PubMed] [Google Scholar]
- ROSZKOWSKI A. P. An unusual type of sympathetic ganglionic stimulant. J Pharmacol Exp Ther. 1961 May;132:156–170. [PubMed] [Google Scholar]
- Saunders J., Showell G. A., Baker R., Freedman S. B., Hill D., McKnight A., Newberry N., Salamone J. D., Hirshfield J., Springer J. P. Synthesis and characterization of all four isomers of the muscarinic agonist 2'-methylspiro[1-azabicyclo[2.2.2]octane-3,4'-[1,3]dioxolane]. J Med Chem. 1987 Jun;30(6):969–975. doi: 10.1021/jm00389a003. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Gillard M., Robberecht P., Christophe J. Kinetic studies of [3H]-N-methylscopolamine binding to muscarinic receptors in the rat central nervous system: evidence for the existence of three classes of binding sites. Mol Pharmacol. 1986 Oct;30(4):305–314. [PubMed] [Google Scholar]
- Watson M., Yamamura H. I., Roeske W. R. [3H]pirenzepine and (-)-[3H]quinuclidinyl benzilate binding to rat cerebral cortical and cardiac muscarinic cholinergic sites. I. Characterization and regulation of agonist binding to putative muscarinic subtypes. J Pharmacol Exp Ther. 1986 May;237(2):411–418. [PubMed] [Google Scholar]


