Abstract
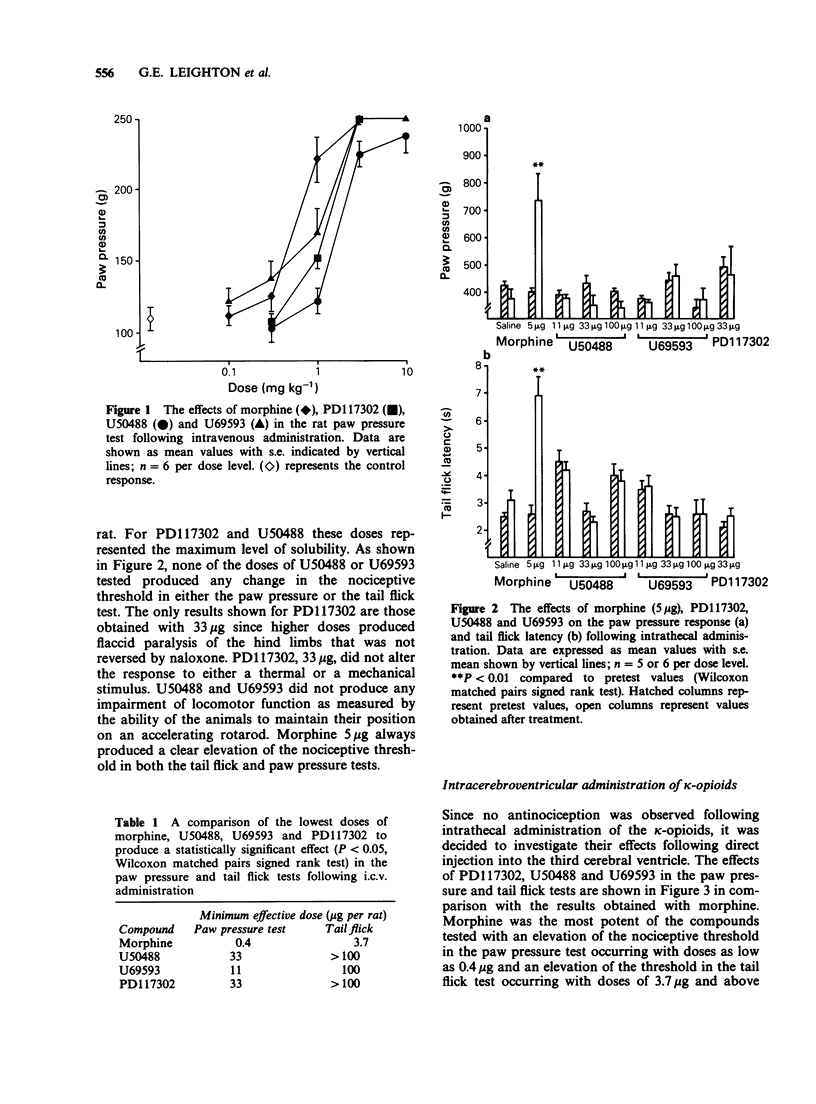
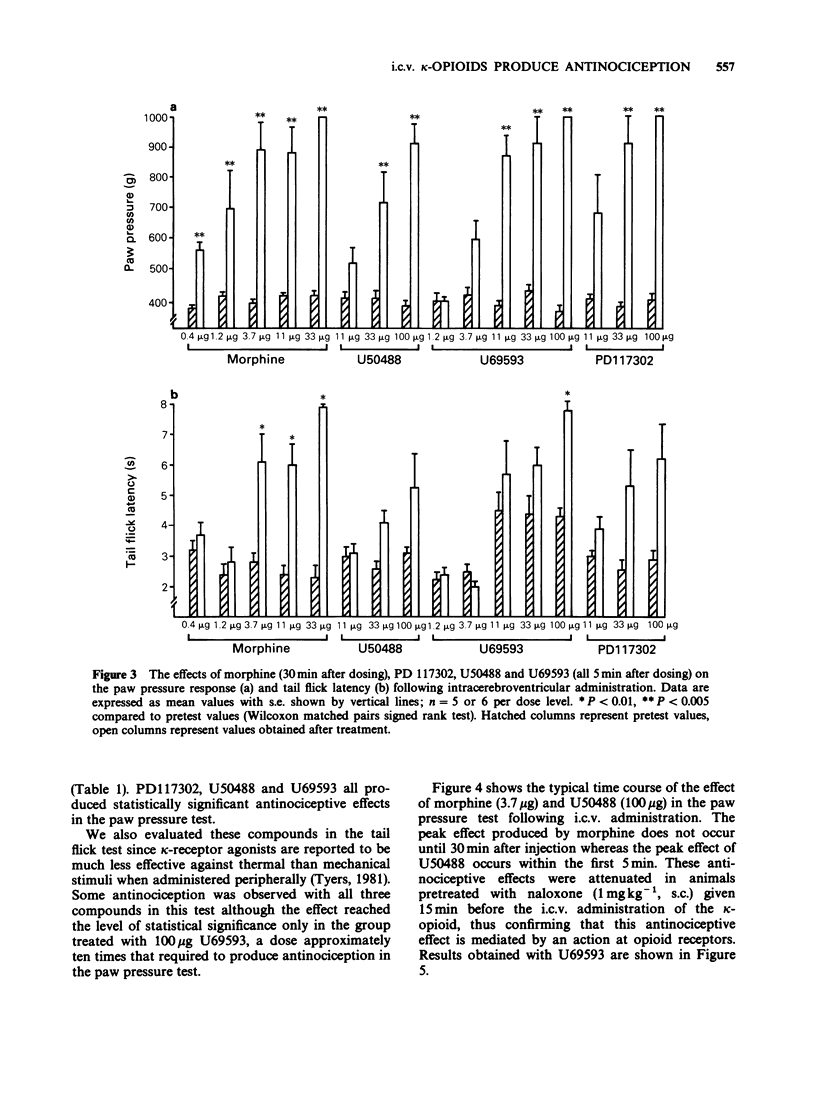
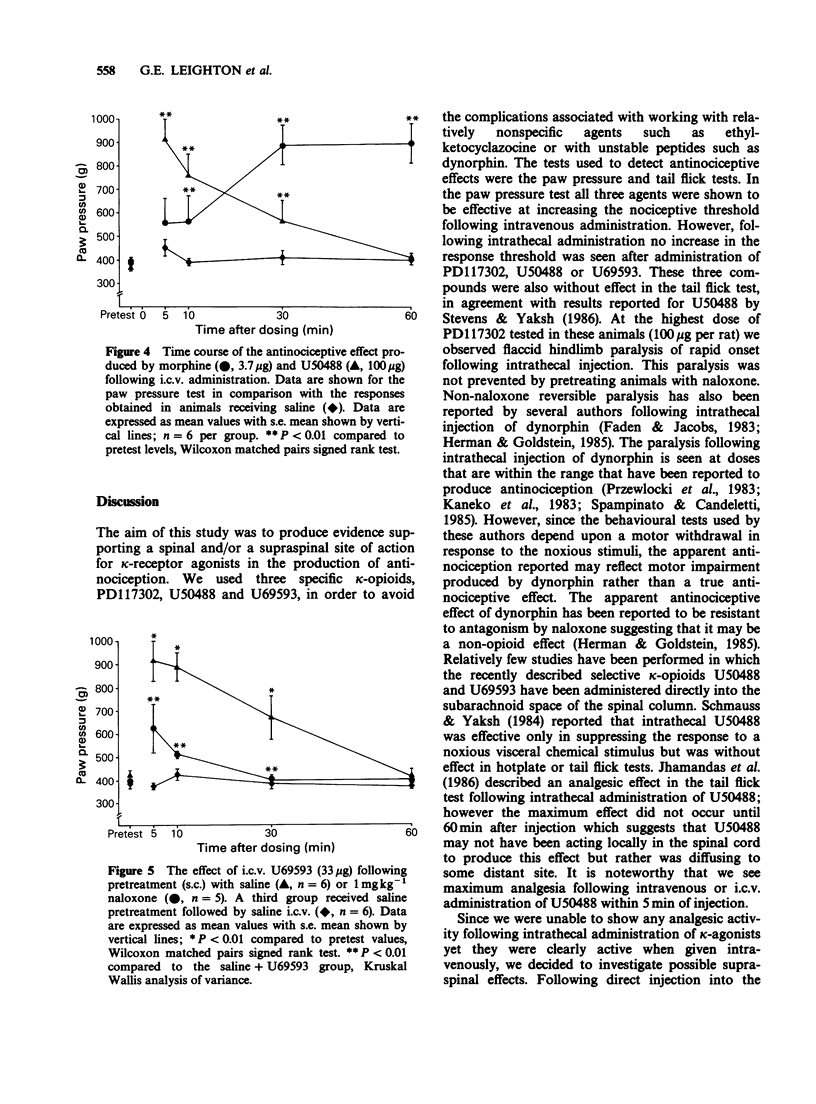
1. Nociceptive thresholds to noxious mechanical (paw pressure) and thermal (tail flick) stimuli were recorded in conscious rats. The effects of three selective kappa-opioid receptor agonists on the responses to these stimuli were determined following intravenous, intracerebroventricular or intrathecal administration. Results were compared with those obtained with morphine. 2. Following intravenous administration PD117302, U50488, U69593 and morphine produced steep parallel dose-response curves indicating antinociceptive activity when evaluated in the paw pressure test. When U50488 and U69593 were tested at a single dose of 3.3 mg kg-1 no effect was seen in the tail flick test. 3. When given by the intrathecal route only morphine was effective at increasing the nociceptive threshold. PD117302, U50488 and U69593 were without effect in either the paw pressure or tail flick tests when tested at doses up to 100 micrograms per rat. PD117302 caused flaccid paralysis of the hindlimbs following intrathecal administration at the top dose tested. This effect was not reversible by naloxone. 4. All three kappa-opioid receptor agonists produced naloxone-reversible antinociception in the paw pressure test, and to a lesser extent in the tail flick test, when injected directly into the third cerebral ventricle with the maximum effect occurring between 5 and 10 min after administration and declining back to control levels by 60 min. Morphine had a much slower onset of action with the peak effect being observed 30 min after dosing. 5. It is concluded that, under our experimental conditions in the rat, the antinociceptive effects of kappa-agonists are likely to be operated via an action at a supraspinal rather than a spinal site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res. 1977 Mar 18;124(1):53–67. doi: 10.1016/0006-8993(77)90863-0. [DOI] [PubMed] [Google Scholar]
- Botticelli L. J., Cox B. M., Goldstein A. Immunoreactive dynorphin in mammalian spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7783–7786. doi: 10.1073/pnas.78.12.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calthrop J., Hill R. G. The action of K-agonists on the nociceptive responses of neurones in the medullary dorsal horn of the anaesthetized rat. Life Sci. 1983;33 (Suppl 1):541–544. doi: 10.1016/0024-3205(83)90560-x. [DOI] [PubMed] [Google Scholar]
- Chavkin C., James I. F., Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982 Jan 22;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Paterson S. J., McKnight A. T., Magnan J., Kosterlitz H. W. Dynorphin and dynorphin are ligands for the kappa-subtype of opiate receptor. Nature. 1982 Sep 2;299(5878):79–81. doi: 10.1038/299079a0. [DOI] [PubMed] [Google Scholar]
- Cowan A., Zhu X. Z., Porreca F. Studies in vivo with ICI 174864 and [D-Pen2, D-Pen5]enkephalin. Neuropeptides. 1985 Feb;5(4-6):311–314. doi: 10.1016/0143-4179(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Faden A. I., Jacobs T. P. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. Br J Pharmacol. 1984 Feb;81(2):271–276. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. L., Emson P. C., Leigh B. K., Gilbert R. F., Iversen L. L. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980 Mar 27;284(5754):351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Gilbert P. E., Martin W. R. The effects of morphine and nalorphine-like drugs in the nondependent, morphine-dependent and cyclazocine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jul;198(1):66–82. [PubMed] [Google Scholar]
- Goldstein A., Fischli W., Lowney L. I., Hunkapiller M., Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H. Kappa opiate receptors localized by autoradiography to deep layers of cerebral cortex: relation to sedative effects. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5703–5707. doi: 10.1073/pnas.79.18.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. G., Skingle M., Tyers M. B. Antinociceptive profile of dynorphin in the rat. Life Sci. 1983;33 (Suppl 1):657–660. doi: 10.1016/0024-3205(83)90588-x. [DOI] [PubMed] [Google Scholar]
- Herman B. H., Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985 Jan;232(1):27–32. [PubMed] [Google Scholar]
- Herz A., Albus K., Metys J., Schubert P., Teschemacher H. On the central sites for the antinociceptive action of morphine and fentanyl. Neuropharmacology. 1970 Nov;9(6):539–551. doi: 10.1016/0028-3908(70)90004-3. [DOI] [PubMed] [Google Scholar]
- Hiller J. M., Simon E. J. Specific, high affinity [3H]ethylketocyclazocine binding in rat central nervous system: lack of evidence for kappa receptors. J Pharmacol Exp Ther. 1980 Sep;214(3):516–519. [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Yoshimura K., Lee N. M., Loh H. H., Way E. L. Dynorphin interaction at the kappa-opiate site. Eur J Pharmacol. 1981 Jun 19;72(2-3):265–266. doi: 10.1016/0014-2999(81)90284-3. [DOI] [PubMed] [Google Scholar]
- James I. F., Chavkin C., Goldstein A. Preparation of brain membranes containing a single type of opioid receptor highly selective for dynorphin. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7570–7574. doi: 10.1073/pnas.79.23.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas K., Sutak M., Lemaire S. Comparative spinal analgesic action of dynorphin1-8, dynorphin1-13, and a kappa-receptor agonist U50,488. Can J Physiol Pharmacol. 1986 Mar;64(3):263–268. doi: 10.1139/y86-042. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Nakazawa T., Ikeda M., Yamatsu K., Iwama T., Wada T., Satoh M., Takagi H. Sites of analgesic action of dynorphin. Life Sci. 1983;33 (Suppl 1):661–664. doi: 10.1016/0024-3205(83)90589-1. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Lamotte C., Pert C. B., Snyder S. H. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976 Aug 13;112(2):407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- Leighton G. E., Johnson M. A., Meecham K. G., Hill R. G., Hughes J. Pharmacological profile of PD 117302, a selective kappa-opioid agonist. Br J Pharmacol. 1987 Dec;92(4):915–922. doi: 10.1111/j.1476-5381.1987.tb11398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Pert A., Yaksh T. Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res. 1974 Nov 8;80(1):135–140. doi: 10.1016/0006-8993(74)90731-8. [DOI] [PubMed] [Google Scholar]
- Przewłocki R., Stala L., Greczek M., Shearman G. T., Przewłocka B., Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life Sci. 1983;33 (Suppl 1):649–652. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- Quirion R., Weiss A. S., Pert C. B. Comparative pharmacological properties and autoradiographic distribution of [3H]ethylketocyclazocine binding sites in rat and guinea pig brain. Life Sci. 1983;33 (Suppl 1):183–186. doi: 10.1016/0024-3205(83)90473-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. E., Leighton G., Hill R. G., Hughes J. In vivo evidence for spinal delta-opiate receptor operated antinociception. Neuropeptides. 1986 Oct;8(3):221–241. doi: 10.1016/0143-4179(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T. L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):1–12. [PubMed] [Google Scholar]
- Spampinato S., Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. Eur J Pharmacol. 1985 Mar 26;110(1):21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Stevens C. W., Yaksh T. L. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. J Pharmacol Exp Ther. 1986 Sep;238(3):833–838. [PubMed] [Google Scholar]
- Traynor J. R., Kelly P. D., Rance M. J. Multiple opiate binding sites in rat spinal cord. Life Sci. 1982 Sep 20;31(12-13):1377–1380. doi: 10.1016/0024-3205(82)90385-x. [DOI] [PubMed] [Google Scholar]
- Tyers M. B. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980 Jul;69(3):503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald E., Sasson S., Kornetsky C. Evaluation of the supraspinal analgesic activity and abuse liability of ethylketocyclazocine. Eur J Pharmacol. 1987 Jan 20;133(3):275–281. doi: 10.1016/0014-2999(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Vonvoigtlander P. F., Lahti R. A., Ludens J. H. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983 Jan;224(1):7–12. [PubMed] [Google Scholar]
- Wood P. L., Rackham A., Richard J. Spinal analgesia: comparison of the mu agonist morphine and the kappa agonist ethylketazocine. Life Sci. 1981 May 11;28(19):2119–2125. doi: 10.1016/0024-3205(81)90618-4. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976 Dec;17(6):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977 Aug;202(2):411–428. [PubMed] [Google Scholar]


