Abstract
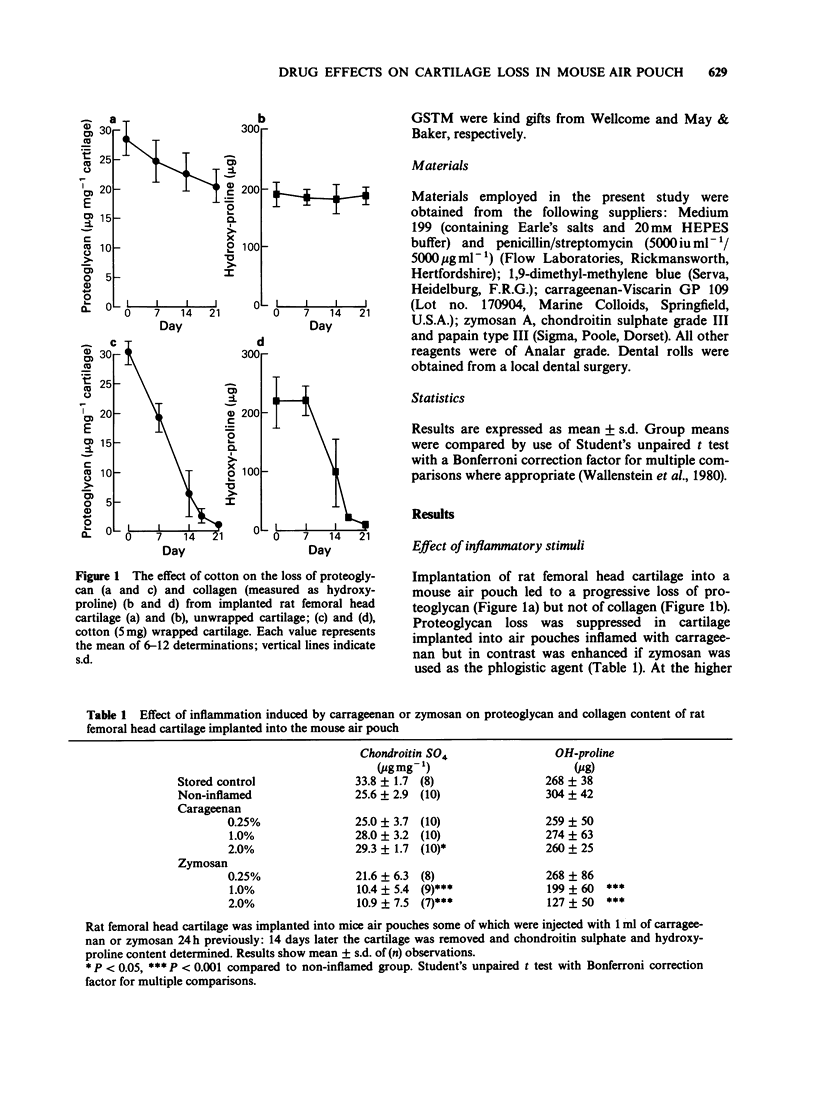
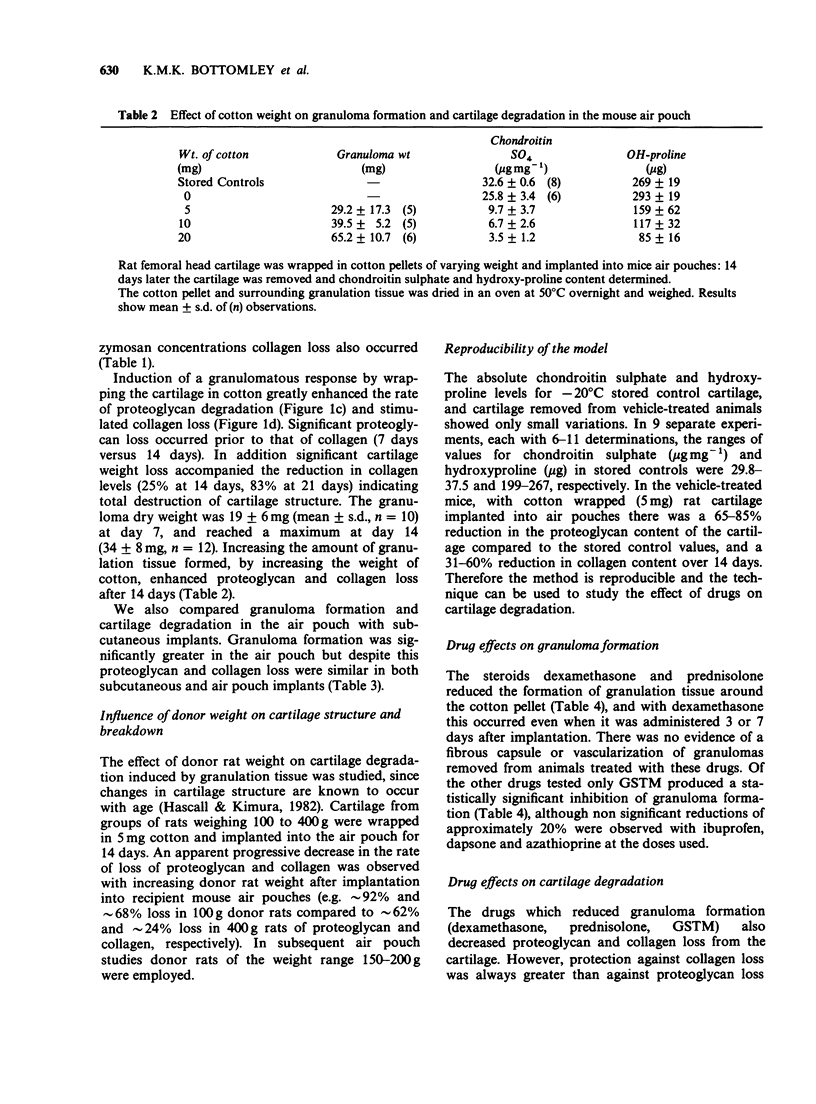
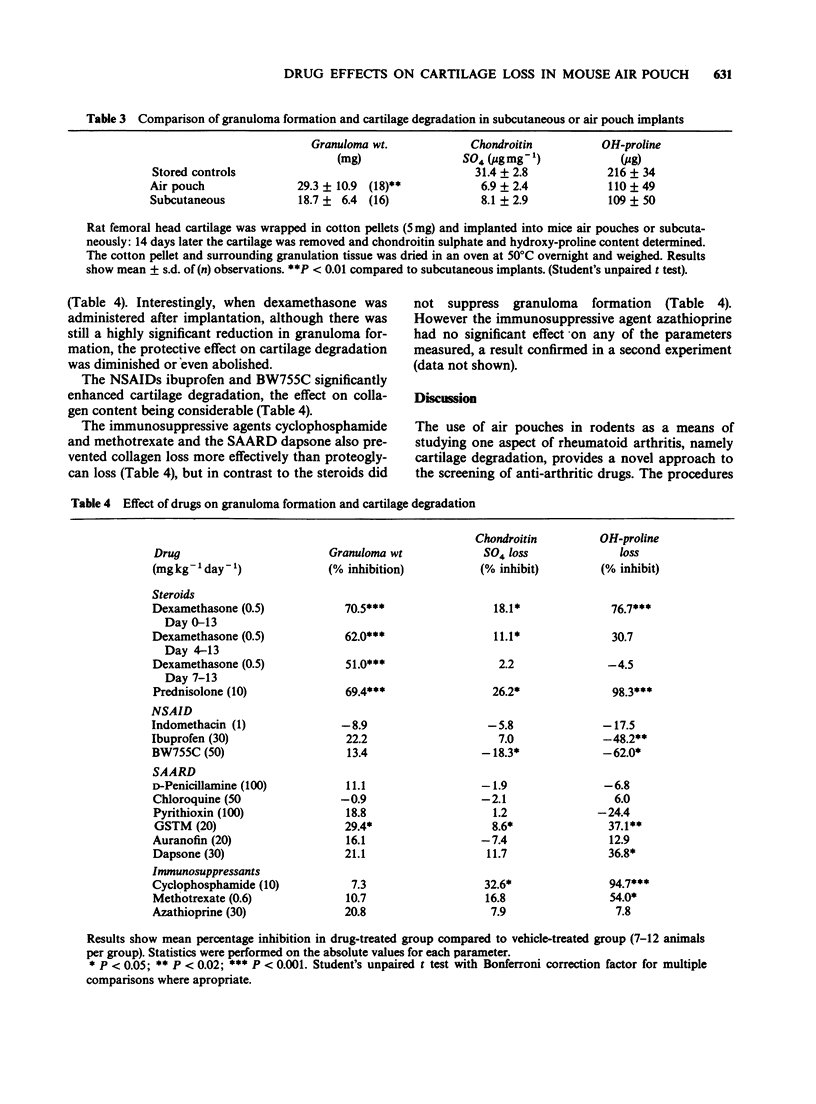
1. Employing rat femoral head cartilage implanted in a 6 day old mouse air pouch, the effects of inflammatory stimuli (i.e. cotton pellets, carrageenan, zymosan) on the loss of proteoglycan and collagen and granuloma formation have been studied. 2. Wrapping of the cartilage in cotton resulted in granuloma formation with accelerated loss of proteoglycan and collagen over the 14 day implantation period. The amount of loss increased with increasing weight of cotton. 3. The effects of different classes of anti-rheumatic drugs on granuloma formation and proteoglycan and collagen loss from cotton wrapped femoral head cartilage in the mouse air pouch have been studied. 4. Non-steroidal anti-inflammatory drugs (NSAIDs) had no influence on granuloma formation, but in general accelerated the rates of proteoglycan and collagen loss. 5. Dexamethasone and prednisolone significantly reduced granuloma formation and had a marked protective effect on cartilage breakdown. 6. Of the slow acting anti-rheumatic drugs examined, only gold sodium thiomalate (GSTM) and dapsone significantly decreased cartilage loss, with an accompanying modest decrease in granuloma formation. 7. The immunosuppressants cyclophosphamide and methotrexate, but not azathioprine, reduced cartilage degradation, but had no effect on granuloma formation. 8. The results for the different classes of anti-inflammatory and anti-rheumatic drugs are discussed in relation to their effects in other animal models and their reported therapeutic activities in man. It is concluded that the mouse air pouch method as described offers advantages as an animal model over existing procedures to predict therapeutic efficacy in man.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigoni-Martelld E., Bramm E. Investigations on the influence of cyclophosphamide, gold sodium thiomalate and D-penicillamine on nystatin oedema and adjuvant arthritis. Agents Actions. 1975 Aug;5(3):264–267. doi: 10.1007/BF02026441. [DOI] [PubMed] [Google Scholar]
- Berg R. A. Determination of 3- and 4-hydroxyproline. Methods Enzymol. 1982;82(Pt A):372–398. doi: 10.1016/0076-6879(82)82074-0. [DOI] [PubMed] [Google Scholar]
- Billingham M. E. Models of arthritis and the search for anti-arthritic drugs. Pharmacol Ther. 1983;21(3):389–428. doi: 10.1016/0163-7258(83)90062-1. [DOI] [PubMed] [Google Scholar]
- Blodgett R. C., Jr, Heuer M. A., Pietrusko R. G. Auranofin: a unique oral chrysotherapeutic agent. Semin Arthritis Rheum. 1984 Feb;13(3):255–273. doi: 10.1016/0049-0172(84)90029-5. [DOI] [PubMed] [Google Scholar]
- Bunch T. W., O'Duffy J. D. Disease-modifying drugs for progressive rheumatoid arthritis. Mayo Clin Proc. 1980 Mar;55(3):161–179. [PubMed] [Google Scholar]
- Camp A. V. Penicillamine in the treatment of rheumatoid arthritis. J Rheumatol Suppl. 1981 Jan-Feb;7:103–106. [PubMed] [Google Scholar]
- Catanzaro P. J., Schwartz H. J., Graham R. C., Jr Spectrum and possible mechanism of carrageenan cytotoxicity. Am J Pathol. 1971 Aug;64(2):387–404. [PMC free article] [PubMed] [Google Scholar]
- Dimitriu A., Fauci A. S. Differential sensitivity of human lymphocyte subpopulations to azathioprine. Transplant Proc. 1979 Mar;11(1):878–881. [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Holsapple M. P., Yim G. K. Therapeutic reduction of ongoing carrageenin-induced inflammation by lipoxygenase, but not cyclooxygenase inhibitors. Inflammation. 1984 Sep;8(3):223–230. doi: 10.1007/BF00916412. [DOI] [PubMed] [Google Scholar]
- Hunneyball I. M., Stewart G. A., Stanworth D. R. Effect of D(-)penicillamine on chronic experimental arthritis in rabbits. Ann Rheum Dis. 1977 Aug;36(4):378–380. doi: 10.1136/ard.36.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannuzzi L., Dawson N., Zein N., Kushner I. Does drug therapy slow radiographic deterioration in rheumatoid arthritis? N Engl J Med. 1983 Oct 27;309(17):1023–1028. doi: 10.1056/NEJM198310273091704. [DOI] [PubMed] [Google Scholar]
- Kimura M., Suzuki J., Amemiya K. Mouse granuloma pouch induced by Freund's complete adjuvant with croton oil. J Pharmacobiodyn. 1985 Jun;8(6):393–400. doi: 10.1248/bpb1978.8.393. [DOI] [PubMed] [Google Scholar]
- Kourounakis L., Kapusta M. A. Restoration of diminished T-cell function in adjuvant induced disease by methotrexate: evidence for two populations of splenic T-cell suppressors. J Rheumatol. 1976 Dec;3(4):346–354. [PubMed] [Google Scholar]
- Lewis A. J., Carlson R. P., Chang J., DeLustro F. Effect of gold salts, D-penicillamine and benoxaprofen on type II collagen-induced arthritis in rats. Agents Actions. 1984 Jun;14(5-6):707–714. doi: 10.1007/BF01978912. [DOI] [PubMed] [Google Scholar]
- Lyle W. H. Clinical response in relation to dose of penicillamine in the treatment of rheumatoid arthritis. Int J Clin Pharmacol Res. 1984;4(6):403–408. [PubMed] [Google Scholar]
- O'Duffy J. D., Luthra H. S. Current status of disease-modifying drugs in progressive rheumatoid arthritis. Drugs. 1984 May;27(5):373–377. doi: 10.2165/00003495-198427050-00001. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Chang Y. H. Comparative study of cyclophosphamide, 6-mercaptopurine, azathiopurine and methotrexate. Relative effects on the humoral and the cellular immune response in the mouse. Clin Exp Immunol. 1976 Nov;26(2):346–354. [PMC free article] [PubMed] [Google Scholar]
- Phadke K., Fouts R. L., Parrish J. E., Butler L. D. Evaluation of the effects of various anti-arthritic drugs on type II collagen-induced mouse arthritis model. Immunopharmacology. 1985 Aug;10(1):51–60. doi: 10.1016/0162-3109(85)90059-1. [DOI] [PubMed] [Google Scholar]
- Probert A. W., Jr, Schrier D. J., Gilbertsen R. B. Effects of antiarthritic compounds on type II collagen-induced arthritis in rats. Arch Int Pharmacodyn Ther. 1984 May;269(1):167–176. [PubMed] [Google Scholar]
- Rainsford K. D. Effects of anti-inflammatory drugs on catabolin-induced cartilage destruction in vitro. Int J Tissue React. 1985;7(2):123–126. [PubMed] [Google Scholar]
- Saklatvala J., Dingle J. T. Identification of catabolin, a protein fro synovium which induces degradation of cartilage in organ culture. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1225–1231. doi: 10.1016/0006-291x(80)90082-0. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Moore A. R., Al-Duaij A., Willoughby D. A. Studies into the association between leucocyte accumulation and oedema formation. Agents Actions. 1985 Dec;17(2):209–213. doi: 10.1007/BF01966594. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Moore A. R., Sin Y. M., Al-Duaij A. Y., Landon B., Willoughby D. A. The effect of therapeutic agents on cartilage degradation in-vivo. J Pharm Pharmacol. 1984 Oct;36(10):709–710. doi: 10.1111/j.2042-7158.1984.tb04854.x. [DOI] [PubMed] [Google Scholar]
- Sin Y. M., Sedgwick A. D., Willoughby D. A. Studies on the mechanism of cartilage degradation. J Pathol. 1984 Jan;142(1):23–30. doi: 10.1002/path.1711420107. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Fowler E. F., Pugh-Humphreys R. G. Immunopharmacology of the macrophage-toxic agent carrageenan. Int J Immunopharmacol. 1979;1(4):247–261. doi: 10.1016/0192-0561(79)90001-8. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Walz D. T., DiMartino M. J., Griswold D. E. Comparative pharmacology and biological effects of different gold compounds. J Rheumatol Suppl. 1982 Jul-Aug;8:54–60. [PubMed] [Google Scholar]
- Willkens R. F. Methotrexate treatment of rheumatoid arthritis. Ann Intern Med. 1985 Oct;103(4):612–613. doi: 10.7326/0003-4819-103-4-612. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Al-Duaij A., de Brito F., Sedgwick A. D., Moore A. R., Williams J. D. A hypothesis for the mode of action of anti-rheumatic drugs in a model of cartilage destruction. J Pharm Pharmacol. 1985 Dec;37(12):931–932. doi: 10.1111/j.2042-7158.1985.tb05010.x. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Sedgwick A. D., Giroud J. P., Al-Duaij A. Y., de Brito F. The use of the air pouch to study experimental synovitis and cartilage breakdown. Biomed Pharmacother. 1986;40(2):45–49. [PubMed] [Google Scholar]
- Wright V., Amos R. Do drugs change the course of rheumatoid arthritis? Br Med J. 1980 Apr 5;280(6219):964–966. doi: 10.1136/bmj.280.6219.964-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Bacon P. A., Blake D. R., Scott D. L., Wainwright A. C., Walton K. W. A model of persistent antigen-induced chronic inflammation in the rat air pouch. Br J Exp Pathol. 1984 Apr;65(2):201–214. [PMC free article] [PubMed] [Google Scholar]
- Zinnes H., Sircar J. C., Lindo N., Schwartz M. L., Fabian A. C., Shavel J., Jr, Kasulanis C. F., Genzer J. D., Lutomski C., DiPasquale G. Isoxicam and related 4-hydroxy-N-isoxazolyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxides. Potent nonsteroidal antiinflammatory agents. J Med Chem. 1982 Jan;25(1):12–18. doi: 10.1021/jm00343a003. [DOI] [PubMed] [Google Scholar]


