Abstract
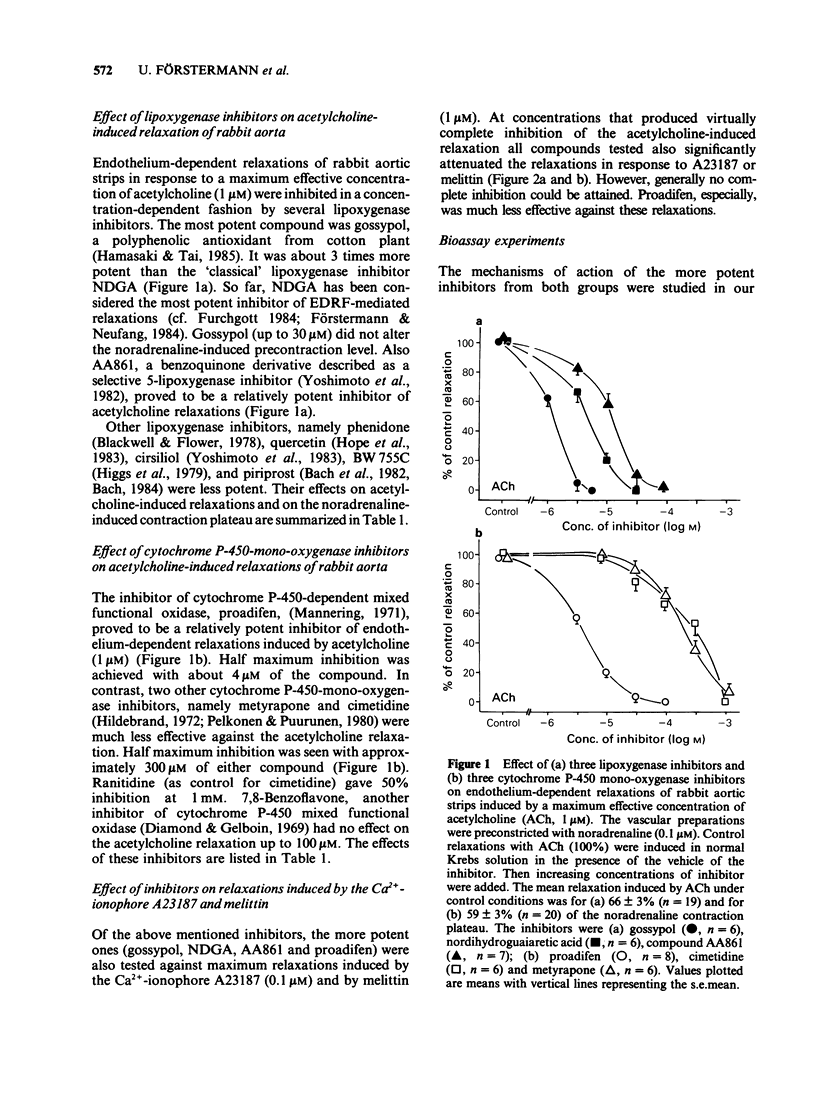
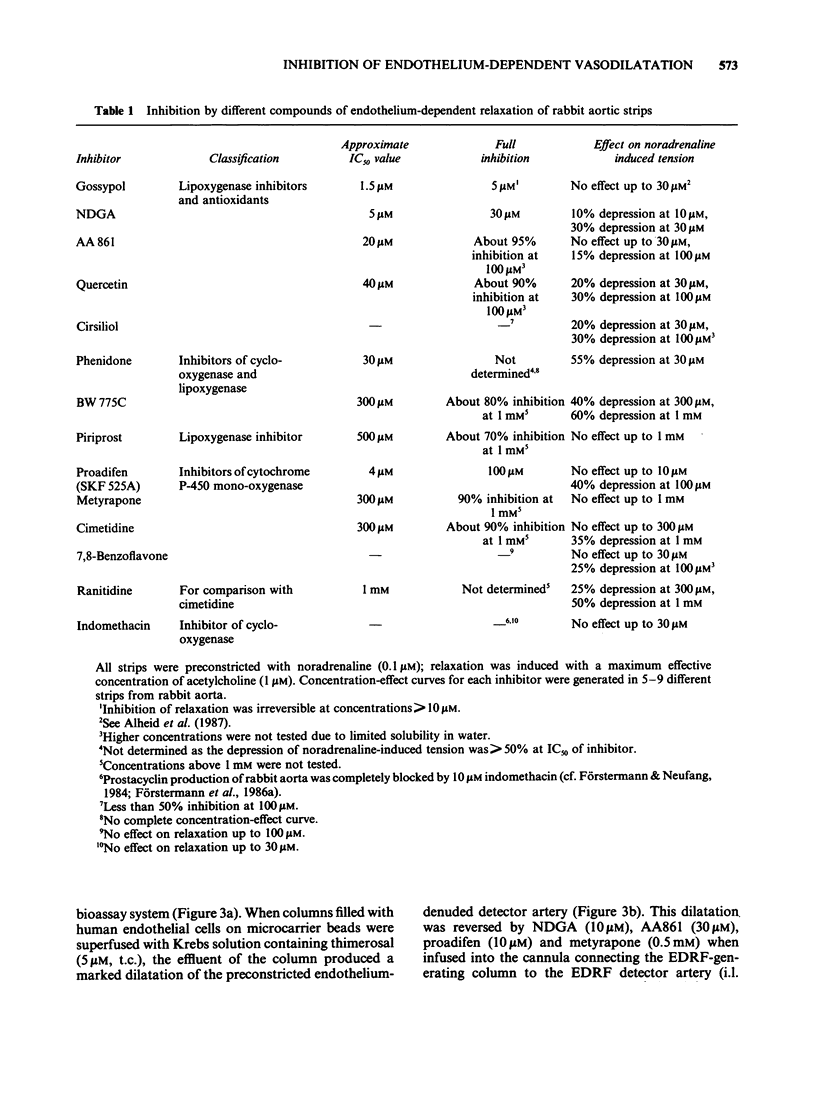
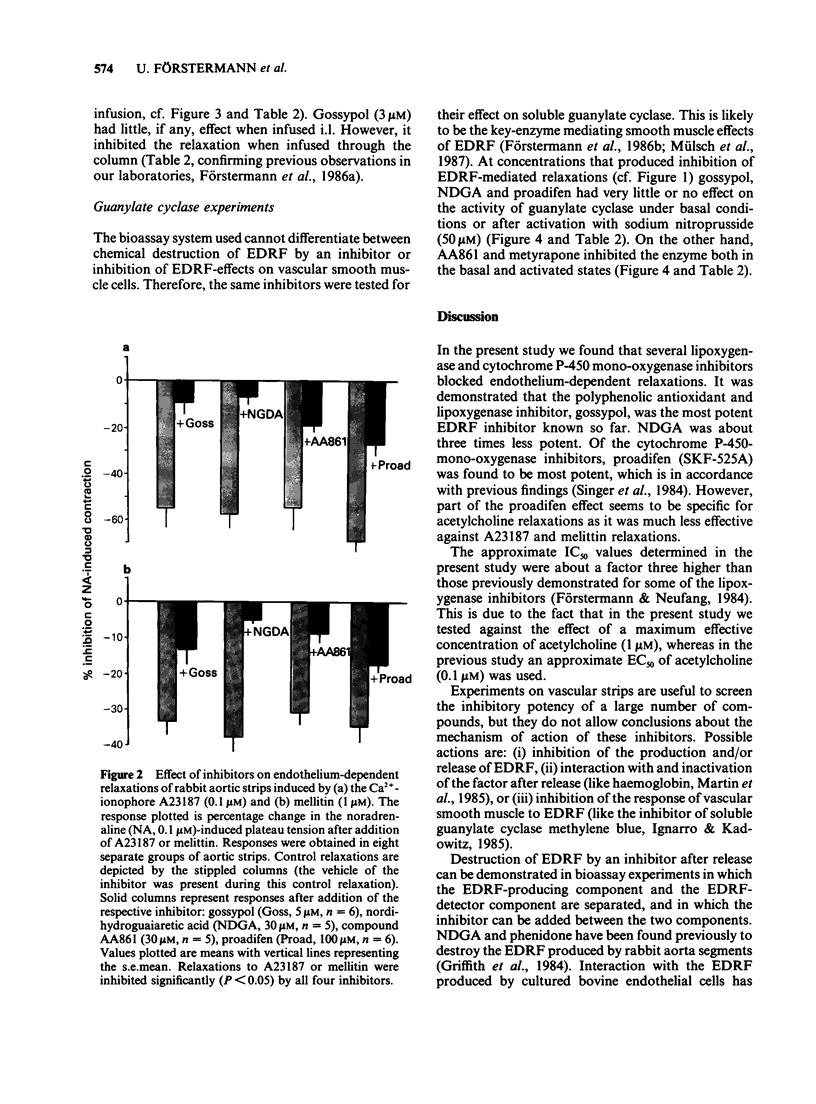
1. Acetylcholine, ionophore A23187 and melittin induced endothelium-dependent relaxations of preconstricted strips of rabbit aorta. These relaxations are likely to be mediated by endothelium-derived relaxing factor (EDRF). 2. Relaxations in response to acetylcholine (1 microM) were inhibited by the following lipoxygenase inhibitors, with the approximate IC50 values indicated in parentheses: gossypol (1.5 microM), nordihydroguairetic acid (NDGA, 5 microM), AA 861 (20 microM), phenidone (30 microM), quercetin (40 microM), BW 755C (300 microM), and piriprost (500 microM); with cirsiliol 50% inhibition was not achieved. Acetylcholine-induced relaxations were also blocked by the cytochrome P-450-mono-oxygenase inhibitors proadifen (SKF 525A, 4 microM), metyrapone (300 microM), and cimetidine (300 microM); 7,8 benzoflavone had no effect up to 100 microM. 3. The more potent inhibitors were also tested against relaxations induced by A23187 (0.1 microM) and melittin (1 microM) and produced partial inhibition of these relaxations. 4. The mechanism of action of the more potent inhibitors was investigated in a bioassay system. EDRF was produced in columns filled with cultured human endothelial cells. The factor was bioassayed with endothelium denuded segments of rabbit femoral artery. When added to effluent of the column, NDGA, AA861, proadifen and metyrapone inhibited the EDRF-induced vasodilatation, whereas gossypol had no effect. Gossypol, however, blocked EDRF production when infused through the column. 5. The more potent inhibitors were also tested to determine their effect on purified soluble guanylate cyclase. While gossypol, NDGA and proadifen had no appreciable effects, basal and nitroprusside (50 microM)-stimulated guanylate cyclase activity was inhibited by AA861 and metyrapone. 6. These data suggest that many of the above compounds inhibit EDRF by mechanisms other than lipoxygenase- or cytochrome P-450-mono-oxygenase inhibition.
Full text
PDF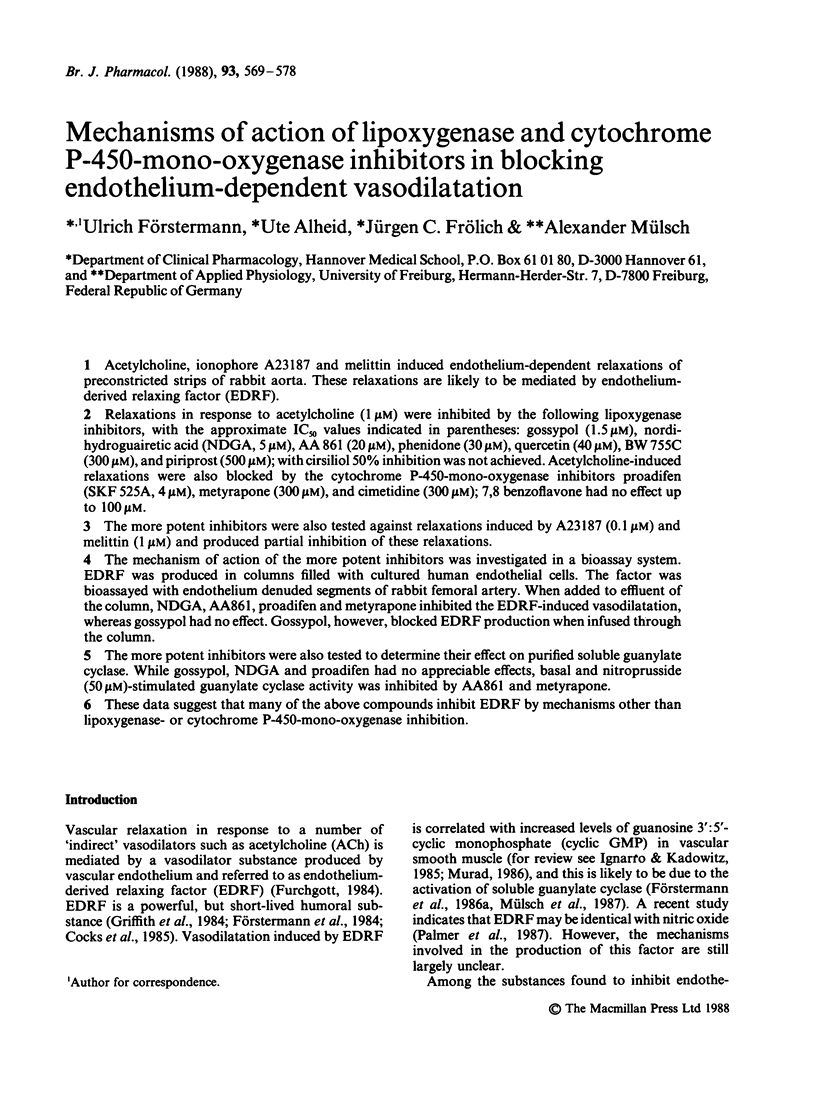


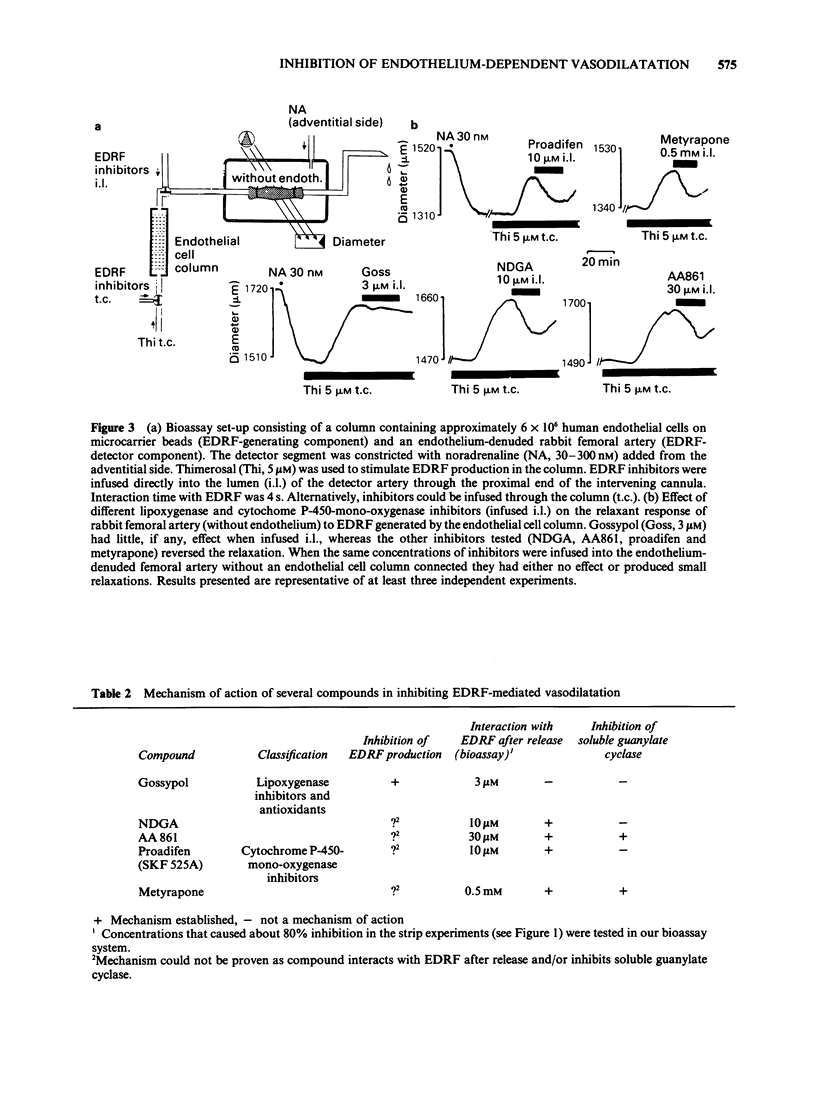
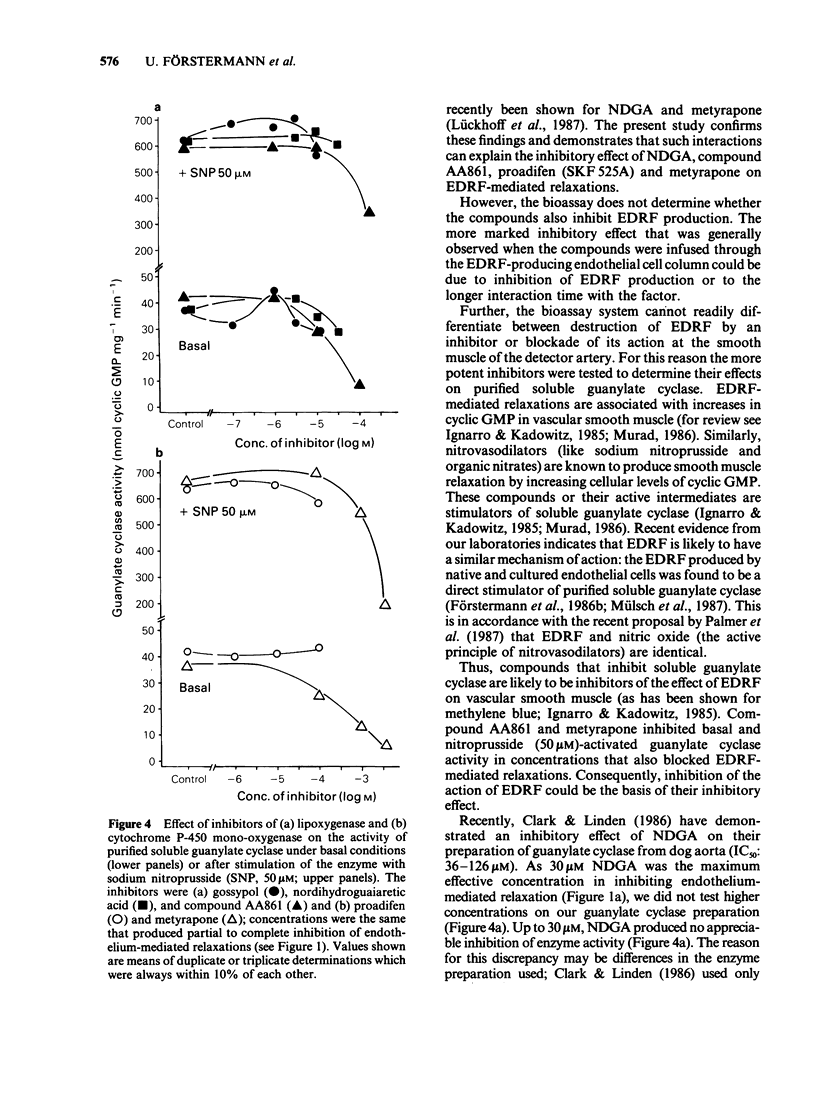


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Pinto A., Mullane K. M., Levere R. D., Spokas E. Presence of cytochrome P-450-dependent monooxygenase in intimal cells of the hog aorta. Hypertension. 1985 Nov-Dec;7(6 Pt 1):899–904. doi: 10.1161/01.hyp.7.6.899. [DOI] [PubMed] [Google Scholar]
- Alheid U., Dudel C., Förstermann U. Selective inhibition by gossypol of endothelium-dependent relaxations augments relaxations to glyceryl trinitrate in rabbit coeliac artery. Br J Pharmacol. 1987 Sep;92(1):237–240. doi: 10.1111/j.1476-5381.1987.tb11317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Smith H. W., Fitzpatrick F. A., Sun F. F., McGuire J. C. 6,9-deepoxy-6,9,-(phenylimino)-delta 6,8-prostaglandin I1, (U-60,257), a new inhibitor of leukotriene C and D synthesis: in vitro studies. Prostaglandins. 1982 May;23(5):759–771. [PubMed] [Google Scholar]
- Bast A., Savenije-Chapel E. M., Kroes B. H. Inhibition of mono-oxygenase and oxidase activity of rat-hepatic cytochrome P-450 by H2-receptor blockers. Xenobiotica. 1984 May;14(5):399–408. doi: 10.3109/00498258409151428. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. 1-phenyl-3-pyrazolidone: an inhibitor of cyclo-oxygenase and lipoxygenase pathways in lung and platelets. Prostaglandins. 1978 Sep;16(3):417–425. doi: 10.1016/0090-6980(78)90220-4. [DOI] [PubMed] [Google Scholar]
- Breen K. J., Bury R., Desmond P. V., Mashford M. L., Morphett B., Westwood B., Shaw R. G. Effects of cimetidine and ranitidine on hepatic drug metabolism. Clin Pharmacol Ther. 1982 Mar;31(3):297–300. doi: 10.1038/clpt.1982.36. [DOI] [PubMed] [Google Scholar]
- Clark D. L., Linden J. Modulation of guanylate cyclase by lipoxygenase inhibitors. Hypertension. 1986 Oct;8(10):947–950. doi: 10.1161/01.hyp.8.10.947. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Diamond L., Gelboin H. V. Alpha-naphthoflavone: an inhibitor of hydrocarbon cytotoxicity and microsomal hydroxylase. Science. 1969 Nov 21;166(3908):1023–1025. doi: 10.1126/science.166.3908.1023. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. Endothelium-dependent vasodilation by melittin: are lipoxygenase products involved? Am J Physiol. 1985 Jul;249(1 Pt 2):H14–H19. doi: 10.1152/ajpheart.1985.249.1.H14. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Trogisch G., Busse R. Species-dependent differences in the nature of endothelium-derived vascular relaxing factor. Eur J Pharmacol. 1984 Nov 27;106(3):639–643. doi: 10.1016/0014-2999(84)90071-2. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hofmann F., Schultz G. Purification of a soluble, sodium-nitroprusside-stimulated guanylate cyclase from bovine lung. Eur J Biochem. 1981 Jun 1;116(3):479–486. doi: 10.1111/j.1432-1033.1981.tb05361.x. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y., Tai H. H. Gossypol, a potent inhibitor of arachidonate 5- and 12-lipoxygenases. Biochim Biophys Acta. 1985 Mar 27;834(1):37–41. doi: 10.1016/0005-2760(85)90173-0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Flower R. J., Vane J. R. A new approach to anti-inflammatory drugs. Biochem Pharmacol. 1979 Jun 15;28(12):1959–1961. doi: 10.1016/0006-2952(79)90651-8. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G. The binding of metyrapone to cytochrome P-450 and its inhibitory action on microsomal hepatic mixed function oxidation reactions. Biochem Soc Symp. 1972;34:79–102. [PubMed] [Google Scholar]
- Hope W. C., Welton A. F., Fiedler-Nagy C., Batula-Bernardo C., Coffey J. W. In vitro inhibition of the biosynthesis of slow reacting substance of anaphylaxis (SRS-A) and lipoxygenase activity by quercetin. Biochem Pharmacol. 1983 Jan 15;32(2):367–371. doi: 10.1016/0006-2952(83)90569-5. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R., Winter I., Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987 Mar;9(3):295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Puurunen J. The effect of cimetidine on in vitro and in vivo microsomal drug metabolism in the rat. Biochem Pharmacol. 1980 Nov 15;29(22):3075–3080. doi: 10.1016/0006-2952(80)90448-7. [DOI] [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Cytochrome P-450-dependent monooxygenase activity and endothelial-dependent relaxations induced by arachidonic acid. J Pharmacol Exp Ther. 1986 Feb;236(2):445–451. [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Endothelium-dependent relaxation of rabbit aorta. II. Inhibition of relaxation stimulated by methacholine and A23187 with antagonists of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983 Sep;226(3):796–801. [PubMed] [Google Scholar]
- Singer H. A., Saye J. A., Peach M. J. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels. 1984;21(5):223–230. doi: 10.1159/000158515. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Yoshimoto T., Furukawa M., Horie T., Watanabe-Kohno S. Arachidonate 5-lipoxygenase and its new inhibitors. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):349–352. doi: 10.1016/0091-6749(84)90128-3. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Furukawa M., Yamamoto S., Horie T., Watanabe-Kohno S. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 1983 Oct 31;116(2):612–618. doi: 10.1016/0006-291x(83)90568-5. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Yokoyama C., Ochi K., Yamamoto S., Maki Y., Ashida Y., Terao S., Shiraishi M. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim Biophys Acta. 1982 Nov 12;713(2):470–473. [PubMed] [Google Scholar]


