Abstract
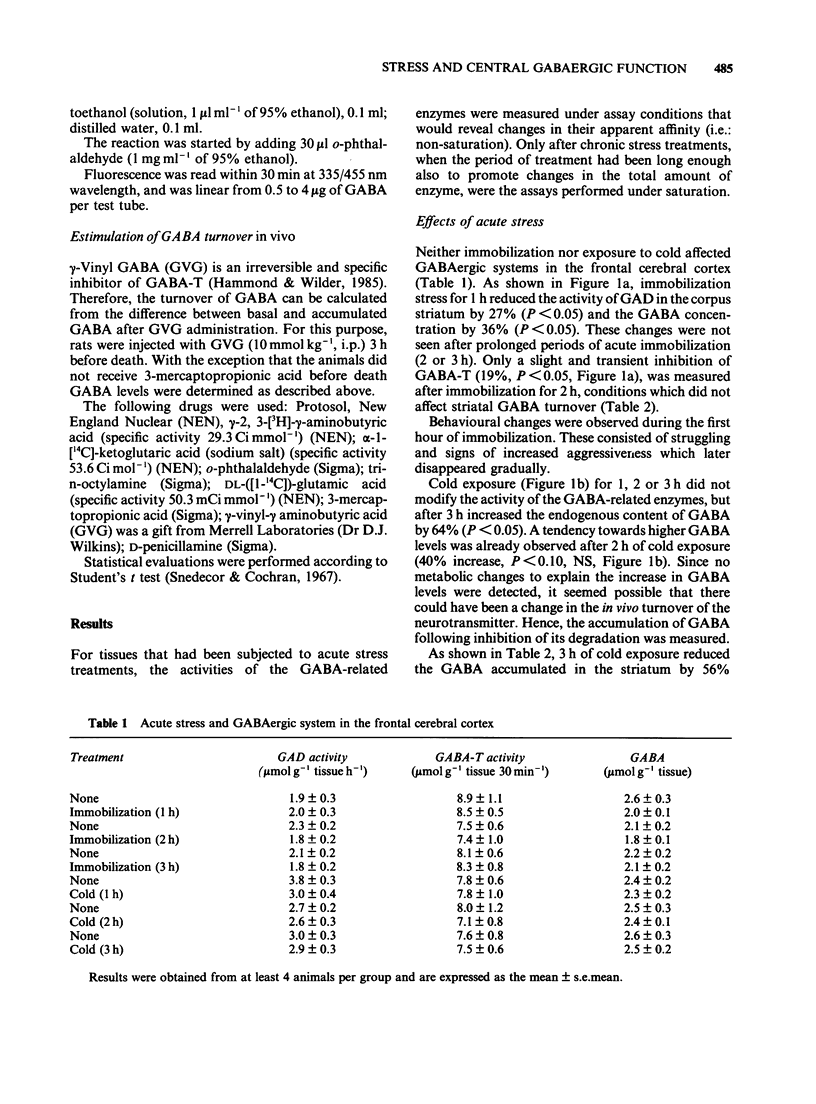
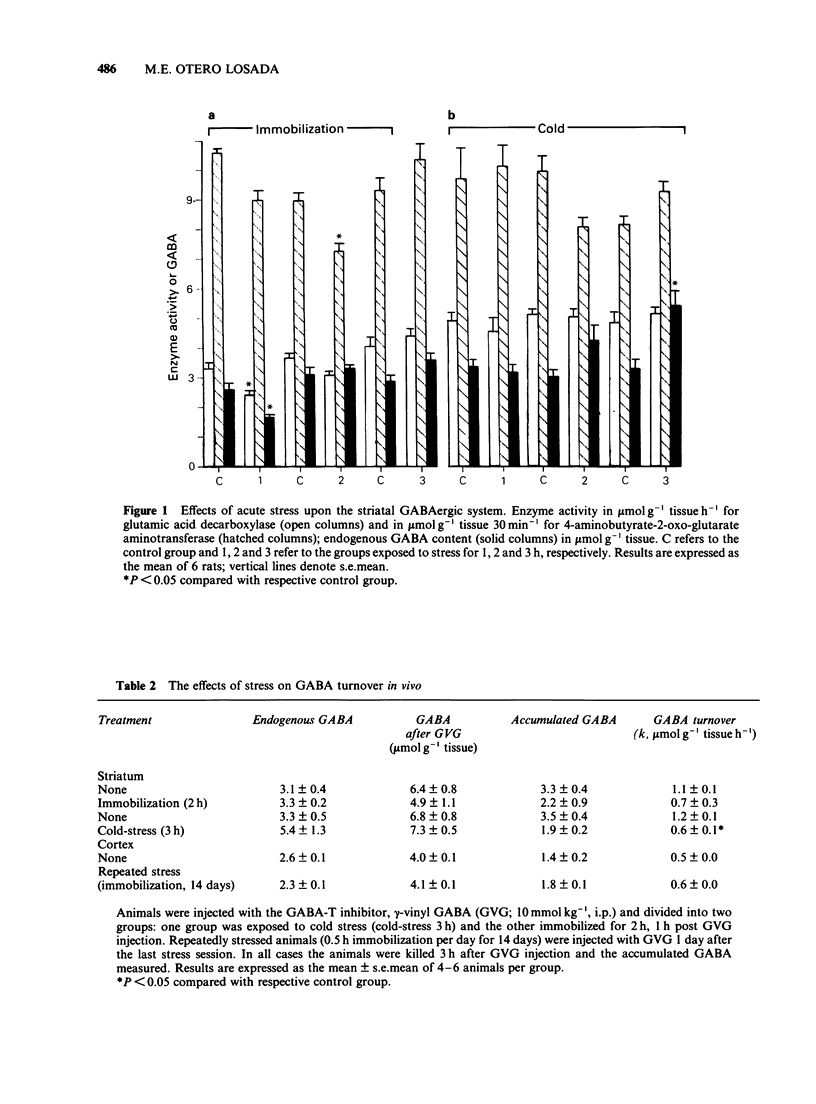
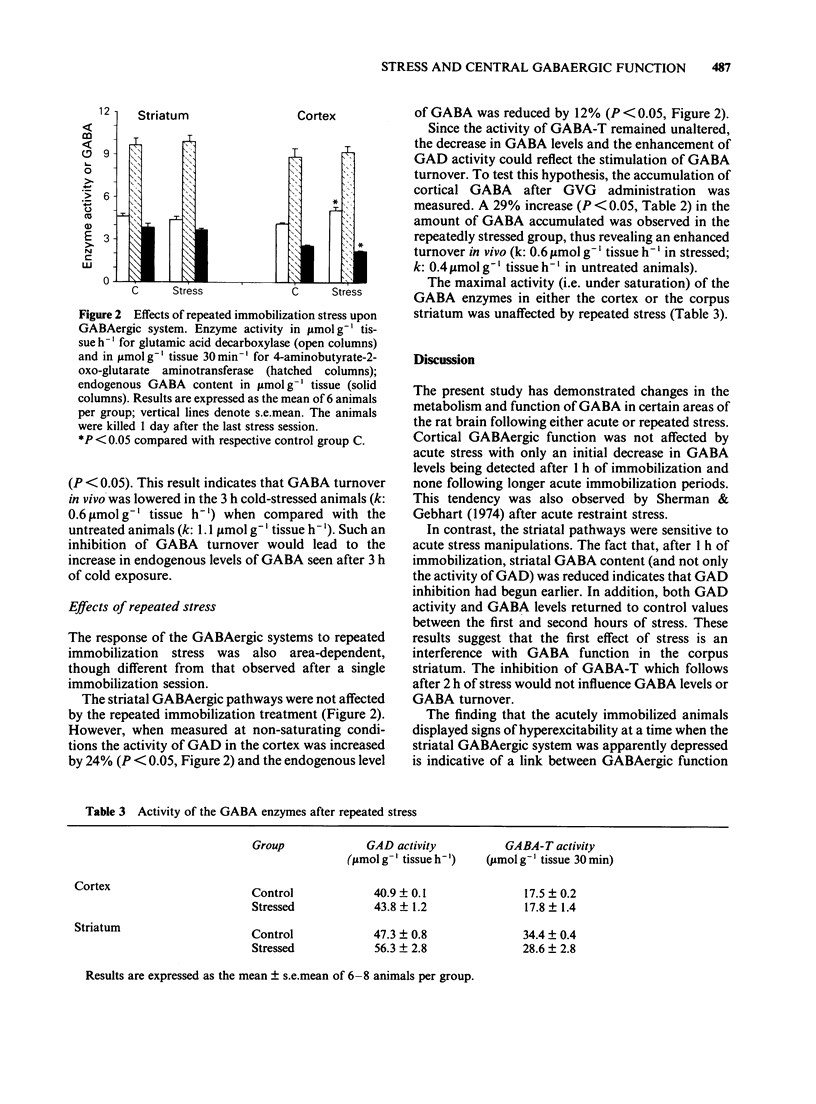
1. The function of gamma-aminobutyric acid (GABA)ergic systems in response to acute and repeated stressful manipulations was evaluated in both the corpus striatum and frontal cerebral cortex of the rat. 2. In the corpus striatum the activity of the synthetic enzyme for GABA (glutamic acid decarboxylase, GAD) and the levels of GABA were reduced by acute immobilization stress (1 h). GABA turnover was reduced only by acute cold stress (3 h, 4 degrees C). 3. In the frontal cerebral cortex no changes were observed after acute stressful manipulations, but repeated stress (0.5 h immobilization per day for 14 days) enhanced both GAD activity and GABA turnover, and reduced GABA levels. 4. In conclusion, it would appear that the GABAergic system in the corpus striatum of the rat is most sensitive to acute stress and that the system in the frontal cerebral cortex area is preferentially responsive to chronic stress. It is speculated that the cortical GABAergic system is responsible for adaptive responses to the adverse conditions prevailing during chronic stress.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERS R. W., BRADY R. O. The distribution of glutamic decarboxylase in the nervous system of the rhesus monkey. J Biol Chem. 1959 Apr;234(4):926–928. [PubMed] [Google Scholar]
- Appelgren B., Eriksson S., Sjöstrand N. O. Commentary on the reduced urinary noradrenaline excretion following cold stress and exercise in physically trained rats. Acta Physiol Scand. 1982 Apr;114(4):579–585. doi: 10.1111/j.1748-1716.1982.tb07027.x. [DOI] [PubMed] [Google Scholar]
- Blanc G., Hervé D., Simon H., Lisoprawski A., Glowinski J., Tassin J. P. Response to stress of mesocortico-frontal dopaminergic neurones in rats after long-term isolation. Nature. 1980 Mar 20;284(5753):265–267. doi: 10.1038/284265a0. [DOI] [PubMed] [Google Scholar]
- Bliss E. L., Ailion J., Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J Pharmacol Exp Ther. 1968 Nov;164(1):122–134. [PubMed] [Google Scholar]
- Corrodi H., Fuxe K., Hökfelt T. The effect of immobilization stress on the activity of central monoamine neurons. Life Sci. 1968 Jan 1;7(1):107–112. doi: 10.1016/0024-3205(68)90368-8. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Jones M. T. Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone ssecretion on subsequent stress responses in the rat. Endocrinology. 1973 May;92(5):1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- Dunn A. J., File S. E. Cold restraint alters dopamine metabolism in frontal cortex, nucleus accumbens and neostriatum. Physiol Behav. 1983 Oct;31(4):511–513. doi: 10.1016/0031-9384(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Fadda F., Argiolas A., Melis M. R., Tissari A. H., Onali P. L., Gessa G. L. Stress-induced increase in 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and in n. accumbens: reversal by diazepam. Life Sci. 1978 Nov 27;23(22):2219–2224. doi: 10.1016/0024-3205(78)90207-2. [DOI] [PubMed] [Google Scholar]
- Glavin G. B., Tanaka M., Tsuda A., Kohno Y., Hoaki Y., Nagasaki N. Regional rat brain noradrenaline turnover in response to restraint stress. Pharmacol Biochem Behav. 1983 Aug;19(2):287–290. doi: 10.1016/0091-3057(83)90054-0. [DOI] [PubMed] [Google Scholar]
- Gold B. I., Bowers M. B., Jr, Roth R. H., Sweeney D. W. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry. 1980 Mar;137(3):362–364. doi: 10.1176/ajp.137.3.362. [DOI] [PubMed] [Google Scholar]
- Green A. R., Peralta E., Hong J. S., Mao C. C., Atterwill C. K., Costa E. Alterations in GABA metabolism and Met-enkephalin content in rat brain following repeated electroconvulsive shocks. J Neurochem. 1978 Sep;31(3):607–611. doi: 10.1111/j.1471-4159.1978.tb07831.x. [DOI] [PubMed] [Google Scholar]
- Haefely W., Kulcsár A., Möhler H., Pieri L., Polc P., Schaffner R. Possible involvement of GABA in the central actions of benzodiazepines. Adv Biochem Psychopharmacol. 1975;(14):131–151. [PubMed] [Google Scholar]
- Hammond E. J., Wilder B. J. Gamma-vinyl GABA. Gen Pharmacol. 1985;16(5):441–447. doi: 10.1016/0306-3623(85)90002-3. [DOI] [PubMed] [Google Scholar]
- Kramarcy N. R., Delanoy R. L., Dunn A. J. Footshock treatment activates catecholamine synthesis in slices of mouse brain regions. Brain Res. 1984 Jan 9;290(2):311–319. doi: 10.1016/0006-8993(84)90949-1. [DOI] [PubMed] [Google Scholar]
- Lavielle S., Tassin J. P., Thierry A. M., Blanc G., Herve D., Barthelemy C., Glowinski J. Blockade by benzodiazepines of the selective high increase in dopamine turnover induced by stress in mesocortical dopaminergic neurons of the rat. Brain Res. 1979 Jun 8;168(3):585–594. doi: 10.1016/0006-8993(79)90311-1. [DOI] [PubMed] [Google Scholar]
- Macdonald R., Barker J. L. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978 Feb 9;271(5645):563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Bisserbe J. C., Eskay R. L. Glucocorticoids are modulators of GABAA receptors in brain. Brain Res. 1985 Jul 22;339(1):178–182. doi: 10.1016/0006-8993(85)90641-9. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Kuwahara T., Sugahara M. Depressive action of gamma-aminobutyraldehyde as a precursor of gamma-aminobutyric acid. Biochem Pharmacol. 1984 Apr 15;33(8):1369–1372. doi: 10.1016/0006-2952(84)90195-3. [DOI] [PubMed] [Google Scholar]
- Petty F., Schlesser M. A. Plasma GABA in affective illness. A preliminary investigation. J Affect Disord. 1981 Dec;3(4):339–343. doi: 10.1016/0165-0327(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Robbins M. S., Hughes J. A., Sparber S. B., Mannering G. J. Delayed teratogenic effect of methylmercury on hepatic cytochrome P-450-dependent monooxygenase systems of rats. Life Sci. 1978 Jan;22(4):287–294. doi: 10.1016/0024-3205(78)90135-2. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. Changes in dopamine, noradrenaline and adrenaline in specific septal and preoptic nuclei after acute immobilization stress. Neuroendocrinology. 1982 Nov;35(5):396–401. doi: 10.1159/000123413. [DOI] [PubMed] [Google Scholar]
- Scheel-Krüger J., Magelund G. GABA in the entopeduncular nucleus and the subthalamic nucleus participates in mediating dopaminergic striatal output functions. Life Sci. 1981 Oct 12;29(15):1555–1562. doi: 10.1016/0024-3205(81)90256-3. [DOI] [PubMed] [Google Scholar]
- Scheel-Krüger J., Magelund G., Olianas M. The role of GABA in the basal ganglia and limbic system for behaviour. Adv Biochem Psychopharmacol. 1981;29:23–36. [PubMed] [Google Scholar]
- Sherman A. D., Petty F. Neurochemical basis of the action of antidepressants on learned helplessness. Behav Neural Biol. 1980 Oct;30(2):119–134. doi: 10.1016/s0163-1047(80)91005-5. [DOI] [PubMed] [Google Scholar]
- Sherman A., Gebhart G. F. Regional levels of GABA and glutamate in mouse brain following exposure to pain. Neuropharmacology. 1974 Jul;13(7):673–675. doi: 10.1016/0028-3908(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Sterri S. H., Fonnum F. Isolation of organic anions by extraction with liquid anion exchangers and its application to micromethods for acetylcholinesterase and 4-aminobutyrate aminotransferase. Eur J Biochem. 1978 Nov 2;91(1):215–222. doi: 10.1111/j.1432-1033.1978.tb20954.x. [DOI] [PubMed] [Google Scholar]
- Stone E. A. Behavioral and neurochemical effects of acute swim stress are due to hypothermia. Life Sci I. 1970 Aug 1;9(15):877–888. doi: 10.1016/0024-3205(70)90050-0. [DOI] [PubMed] [Google Scholar]
- Tarizzo V. I., Rubio M. C. Effects of exercise on several adrenergic system parameters. Acta Physiol Pharmacol Latinoam. 1984;34(1):55–63. [PubMed] [Google Scholar]
- Thierry A. M., Javoy F., Glowinski J., Kety S. S. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968 Sep;163(1):163–171. [PubMed] [Google Scholar]
- Tunnicliff G., Smith J. A. Competitive inhibition of gamma-aminobutyric acid receptor binding by N-2-hydroxyethylpiperazine-N'-2-e-ethanesulfonic acid and related buffers. J Neurochem. 1981 Mar;36(3):1122–1126. doi: 10.1111/j.1471-4159.1981.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Waddington J. L. Behavioural evidence for GABAergic activity of the benzodiazepine flurazepam. Eur J Pharmacol. 1978 Oct 15;51(4):417–422. doi: 10.1016/0014-2999(78)90433-8. [DOI] [PubMed] [Google Scholar]
- Wielosz M., Stelmasiak M., Ossowska G., Kleinrok Z. Effects of electroconvulsive shock on central GABA-ergic mechanisms. Pol J Pharmacol Pharm. 1985 Mar-Apr;37(2):113–122. [PubMed] [Google Scholar]
- Yoneda Y., Kanmori K., Ida S., Kuriyama K. Stress-induced alterations in metabolism of gamma-aminobutyric acid in rat brain. J Neurochem. 1983 Feb;40(2):350–356. doi: 10.1111/j.1471-4159.1983.tb11289.x. [DOI] [PubMed] [Google Scholar]
- Young E. A., Akil H. Corticotropin-releasing factor stimulation of adrenocorticotropin and beta-endorphin release: effects of acute and chronic stress. Endocrinology. 1985 Jul;117(1):23–30. doi: 10.1210/endo-117-1-23. [DOI] [PubMed] [Google Scholar]
- Zacharko R. M., Bowers W. J., Anisman H. Responding for brain stimulation: stress and desmethylimipramine. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8(4-6):601–606. doi: 10.1016/0278-5846(84)90021-6. [DOI] [PubMed] [Google Scholar]
- van der Heyden J. A., Korf J. Regional levels of GABA in the brain: rapid semiautomated assay and prevention of postmortem increase by 3-mercapto-propionic acid. J Neurochem. 1978 Jul;31(1):197–203. doi: 10.1111/j.1471-4159.1978.tb12448.x. [DOI] [PubMed] [Google Scholar]


