Abstract
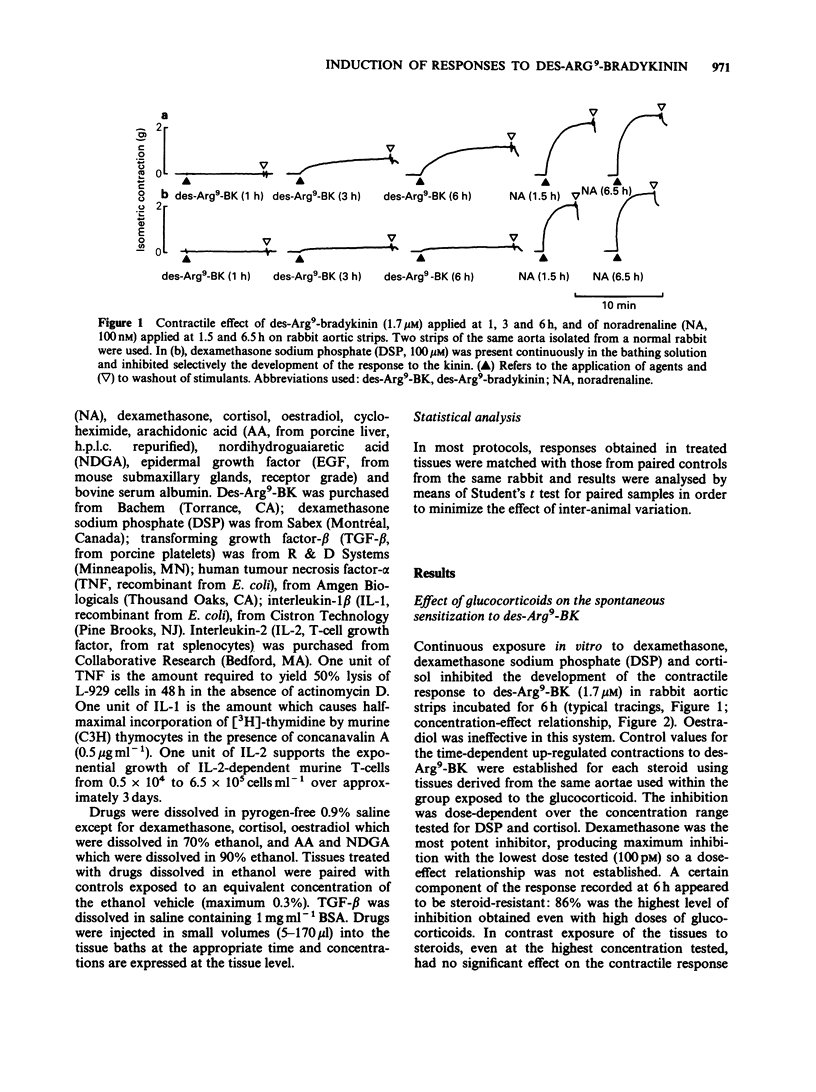
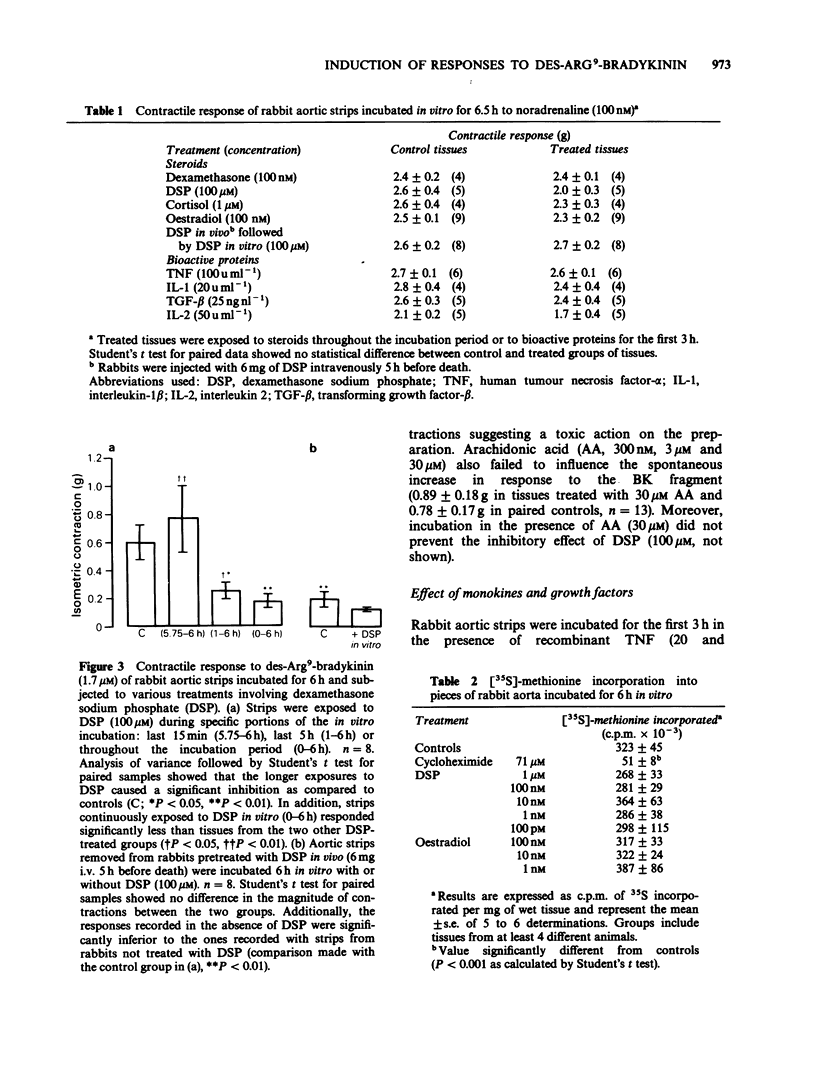
1. The mechanisms modulating the spontaneous induction of contractile responses to agonists of the B1-receptors for kinins have been studied by submitting the rabbit isolated aorta preparation to various in vitro treatments. Des-Arg9-bradykinin (Des-Arg9-BK), applied after 6 h of in vitro incubation was the standard stimulus to monitor this up-regulation process. 2. Specific inhibition of the development of the contractile response to des-Arg9-BK was obtained by exposing tissues continuously to dexamethasone, dexamethasone sodium phosphate (DSP) or cortisol, but not to oestradiol. The maximal extent of the inhibition obtained at high concentrations of glucocorticoids was 86%. 3. No gross inhibition of protein synthesis was observed in the presence of DSP as monitored by [35S]-methionine incorporation into incubated pieces of rabbit aorta. 4. In vivo pretreatment of rabbits with DSP did not reduce further the development of the responses in vitro. DSP applied 15 min before the 6 h recording did not antagonize the contractile effect of the BK fragment. 5. Interleukin 1 (IL-1) and interleukin 2 (IL-2) applied in vitro for the first 3 h of incubation increased the development of the contractile response to des-Arg9-BK. 6. Arachidonic acid (AA), nordihydroguaiaretic acid, tumour necrosis factor-alpha (TNF) and transforming growth factor-beta (TGF-beta) failed to influence the spontaneous development of the response to kinins. 7. Continuous exposure to DSP (100 microM) markedly inhibited the action of stimulants in this system: IL-1, IL-2, epidermal growth factor and muramyl dipeptide. Moreover, the presence of AA (30 microM) did not prevent the inhibitory effect of DSP (100 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
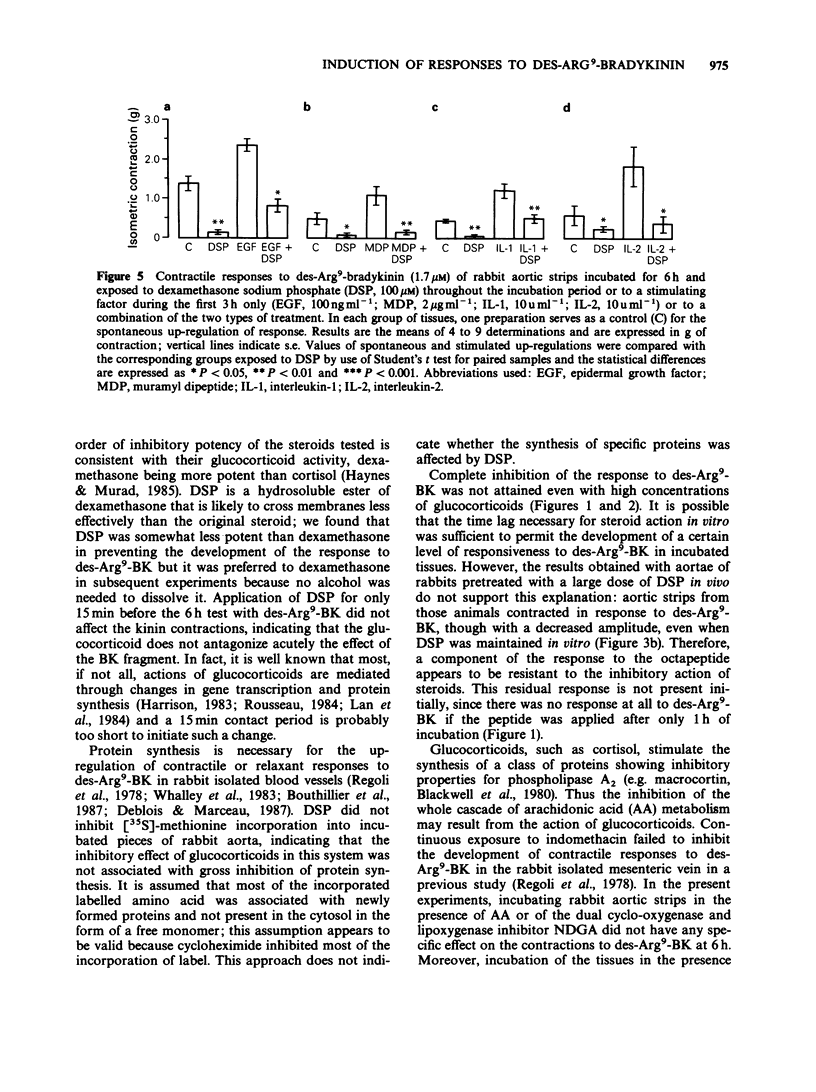
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Marceau F. The ability of des-Arg9-bradykinin to relax rabbit isolated mesenteric arteries is acquired during in vitro incubation. Eur J Pharmacol. 1987 Oct 6;142(1):141–144. doi: 10.1016/0014-2999(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Krueger J. M. Induction of interleukin 1 by synthetic and naturally occurring muramyl peptides. Fed Proc. 1986 Oct;45(11):2545–2548. [PubMed] [Google Scholar]
- Harrison R. W., 3rd Cellular factors which modulate hormone responses: glucocorticoid action in perspective. Int Rev Cytol Suppl. 1983;15:1–16. doi: 10.1016/b978-0-12-364376-6.50007-7. [DOI] [PubMed] [Google Scholar]
- Holter W., Goldman C. K., Casabo L., Nelson D. L., Greene W. C., Waldmann T. A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol. 1987 May 1;138(9):2917–2922. [PubMed] [Google Scholar]
- Iribe H., Koga T., Kotani S., Kusumoto S., Shiba T. Stimulating effect of MDP and its adjuvant-active analogues on guinea pig fibroblasts for the production of thymocyte-activating factor. J Exp Med. 1983 Jun 1;157(6):2190–2195. doi: 10.1084/jem.157.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N. C., Karin M., Nguyen T., Weisz A., Birnbaum M. J., Eberhardt N. L., Baxter J. D. Mechanisms of glucocorticoid hormone action. J Steroid Biochem. 1984 Jan;20(1):77–88. doi: 10.1016/0022-4731(84)90192-4. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Cerottini J. C., MacDonald H. R. Interleukin 1-dependent induction of both interleukin 2 secretion and interleukin 2 receptor expression by thymoma cells. J Immunol. 1986 Aug 15;137(4):1226–1231. [PubMed] [Google Scholar]
- Marceau F., Lussier A., St-Pierre S. Selective induction of cardiovascular responses to des-Arg9-bradykinin by bacterial endotoxin. Pharmacology. 1984;29(2):70–74. doi: 10.1159/000137994. [DOI] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- Náray-Fejes-Tóth A., Fejes-Tóth G., Fischer C., Frölich J. C. Effect of dexamethasone on in vivo prostanoid production in the rabbit. J Clin Invest. 1984 Jul;74(1):120–123. doi: 10.1172/JCI111391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D. C., Marceau F., Lavigne J. Induction of beta 1-receptors for kinins in the rabbit by a bacterial lipopolysaccharide. Eur J Pharmacol. 1981 Apr 24;71(1):105–115. doi: 10.1016/0014-2999(81)90391-5. [DOI] [PubMed] [Google Scholar]
- Regoli D., Marceau F., Barabé J. De novo formation of vascular receptors for bradykinin. Can J Physiol Pharmacol. 1978 Aug;56(4):674–677. doi: 10.1139/y78-109. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G. Control of gene expression by glucocorticoid hormones. Biochem J. 1984 Nov 15;224(1):1–12. doi: 10.1042/bj2240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Liu D. Y. Mononuclear phagocyte activation: activation-associated antigens. Fed Proc. 1986 Nov;45(12):2829–2836. [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Whalley E. T., Fritz H., Geiger R. Kinin receptors and angiotensin converting enzyme in rabbits basilar arteries. Naunyn Schmiedebergs Arch Pharmacol. 1983 Dec;324(4):296–301. doi: 10.1007/BF00502627. [DOI] [PubMed] [Google Scholar]


