Abstract
1. Ca currents in rat and mouse sensory dorsal root ganglion (DRG) neurones were inhibited by concentrations of (-)-baclofen as low as 1 micron. The proportion of neurones responding to baclofen was low (less than 20%), except in young cultures of neonate rat DRG neurones (3 days in culture), where 86% of the neurones were responsive. 2. Three types of unitary Ca currents were observed in the rat DRG neurones, corresponding to the T-, N- and L-type currents of chick DRG neurones. 3. Baclofen produced two types of response on whole-cell currents of DRG neurones from both species. The first was on an early inactivating component of the Ca current. This early current was partially inactivated at a holding potential of -40 mV. It was also reduced during the second of a pair of depolarizing command pulses. The results suggest that this action of baclofen is due to an action on an N-type component of the current. The second response to baclofen persisted throughout the command step and was not reduced during the second of a pair of command pulses, indicating that this effect is due to an action on the L-type current. 4. Unitary or ensemble Ca currents recorded in cell-attached patches, on neurones previously shown to respond to baclofen in whole-cell clamp mode, were generally not inhibited by baclofen application external to the patch electrode. This indicates that a readily diffusible internal second messenger substance is probably not involved in coupling the GABAB receptor to the ion channels.
Full text
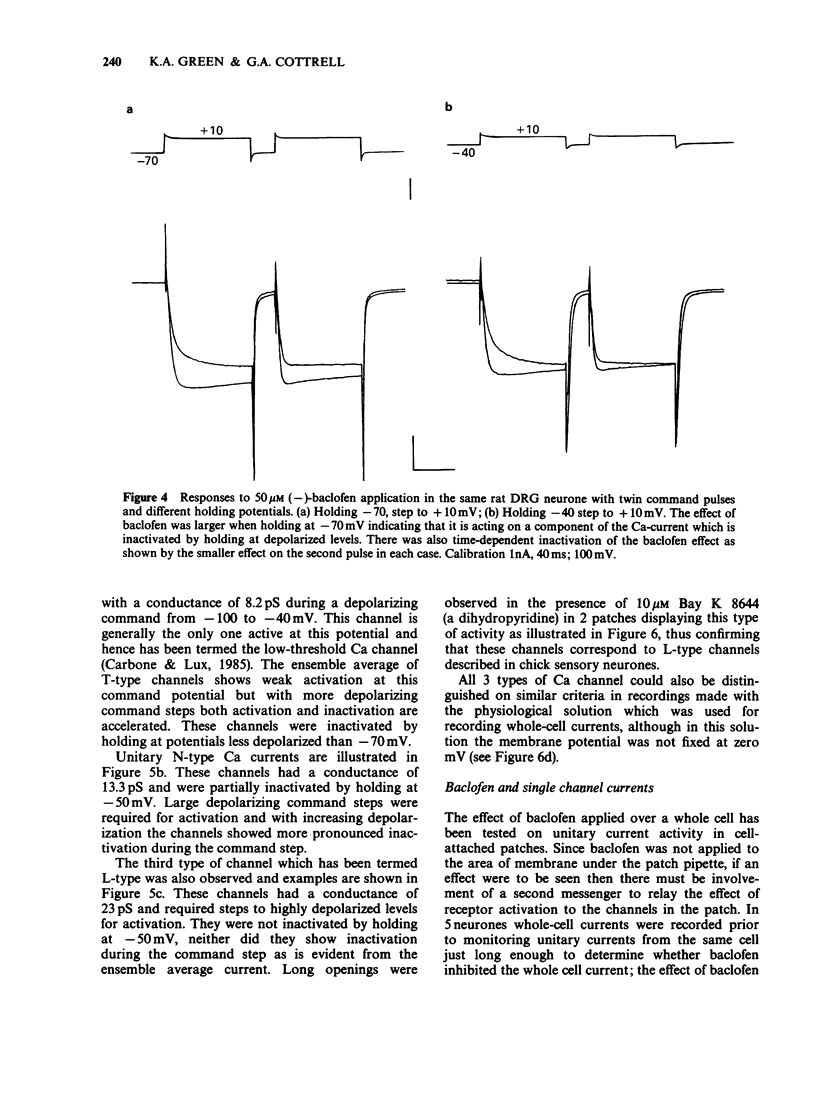
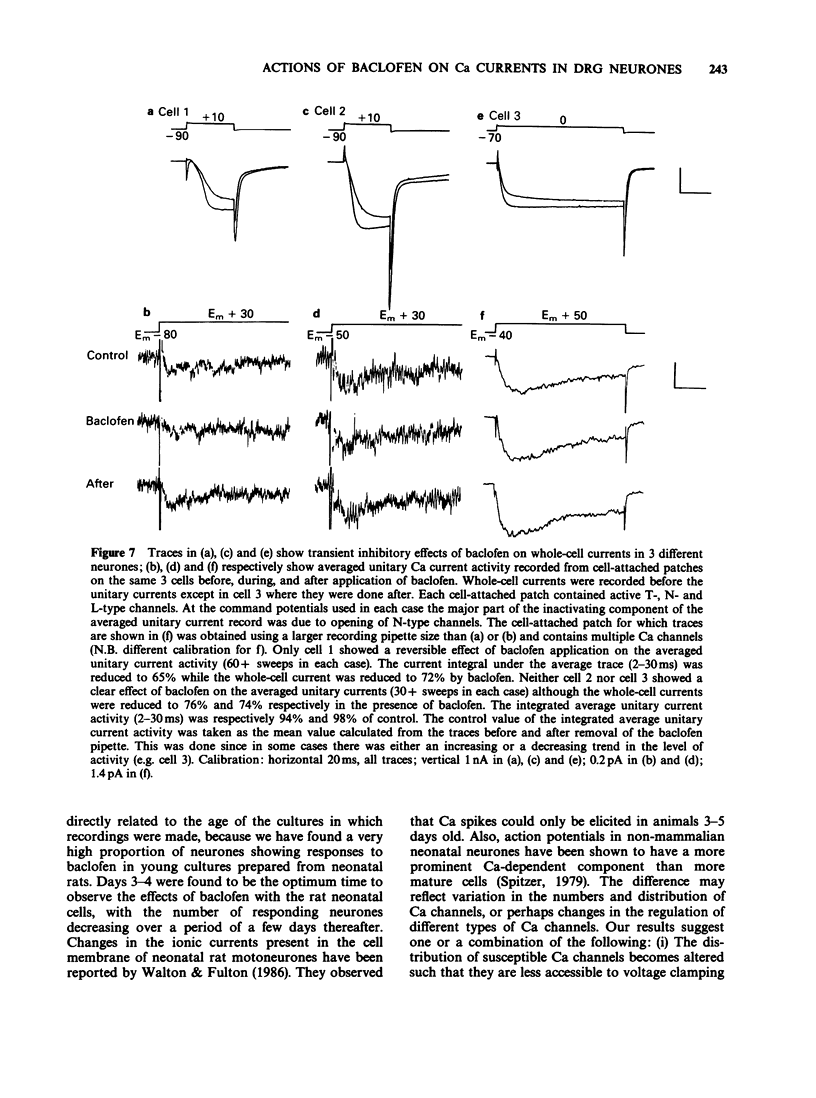
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Functional and pharmacological differences between two types of GABA receptor on embryonic chick sensory neurons. Neurosci Lett. 1984 Jun 29;47(3):265–270. doi: 10.1016/0304-3940(84)90524-x. [DOI] [PubMed] [Google Scholar]
- Désarmenien M., Feltz P., Occhipinti G., Santangelo F., Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. Br J Pharmacol. 1984 Feb;81(2):327–333. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forda S., Kelly J. S. The possible modulation of the development of rat dorsal root ganglion cells by the presence of 5-HT-containing neurones of the brainstem in dissociated cell culture. Brain Res. 1985 Sep;354(1):55–65. doi: 10.1016/0165-3806(85)90068-9. [DOI] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S., Schulz D. Noradrenaline modulates calcium channels in avian dorsal root ganglion cells through tight receptor-channel coupling. J Physiol. 1986 Oct;379:131–144. doi: 10.1113/jphysiol.1986.sp016244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K., Fulton B. P. Ionic mechanisms underlying the firing properties of rat neonatal motoneurons studied in vitro. Neuroscience. 1986 Nov;19(3):669–683. doi: 10.1016/0306-4522(86)90291-5. [DOI] [PubMed] [Google Scholar]


